Gyrolift: A Robotic Wheelchair With Segway Technology
Among Canadians who have a disability, nearly 80% require some type of assistive aid, which helps contribute to more independence and better quality of life. A variety of assistive aids are currently on the market, with requirements for mobility aids dependent upon the individual’s needs and the extent of their disability. Recent advancements have been made to incorporate robotic technology into wheelchairs to allow individuals to become more verticalized (i.e., more upright) and use their lower limbs again, potentially improving a user’s autonomy and quality of life.

This image is an illustration of a grey-and-black two-wheeled wheelchair in an upright position.
(Photo reproduced with permission from Gyrolift.)
How It Works
The Gyrolift is a robotic wheelchair that combines Segway technology and electric verticalization (i.e., when the wheelchair stands).1 Unlike other powered wheelchairs with verticalization capabilities, the Gyrolift allows users to transition continuously into a standing position, even when moving. The wheelchair system is composed of the motor, a verticalization system, and a seat, and has two large wheels (as opposed to other electric wheelchairs that usually have four wheels), which makes the wheelchair more manoeuvrable and compact.2
Who Might Benefit?
In 2017, Statistics Canada conducted a national survey that found that 13.7% of Canadians have a disability that makes daily activities difficult to perform, with mobility being one of the most common issues.3 The Gyrolift has the potential to benefit individuals with paraplegia or those with extensive immobility. As the built environment has been designed for the able-bodied person, the wheelchair’s verticalization feature may improve a user’s psychological well-being, autonomy, and overall quality of life (due to the wheelchair’s unique features and lesser need for the assistance of others).
Availability in Canada
The Gyrolift is not currently available in Canada, the US, France, or other European countries. The manufacturer noted that it expects to start marketing the device in Canada around early 2021, although the device has yet to go through Health Canada for approval (Maxime Giraux, Managing Director, Gyrolift, Paris, France: personal communication, Jan 2020).
What Does It Cost?
The manufacturer stated that the final price of the wheelchair is not yet known but it will be marketed for around €15,000 in France, depending on the chosen model (Maxime Giraux: personal communication, Jan 2020). There is no evidence to suggest that the wheelchair may require set-up costs, special training, or other financial implications to the patient or health care system.
Current Practice
Wheelchairs are common assistive devices for individuals with paraplegia or those with other mobility concerns. They are categorized into manual, electric, reclining, or ergonomic wheelchairs. It is unclear whether there is a standard practice for selecting the appropriate wheelchair or mobility aid for those individuals requiring an assistive device in Canada.
What Is the Evidence?
Currently, no clinical trials have taken place to examine the use of the Gyrolift. The manufacturer noted that it intends to perform future clinical trials to receive regulation and marketing approval in North America. It specified that the clinical trials will likely be conducted in early 2020 (Maxime Giraux: personal communication, Jan 2020).
Other Information
A protocol study2 was published in 2014 that detailed the features and characteristics of the Gyrolift and an early experimental study2 allowing users to test out the wheelchair’s maneuverability and handling. Participants in the study reported that the Gyrolift was user friendly and easy to maneuver; however, they also reported some discomfort and sizing issues with the seat and leg rests. The seat size is important as the user’s chest needs to be in the correct position in order to control the verticalization of the wheelchair. The researchers noted that the next step will be to test out a new and enhanced version of the Gyrolift with a larger panel of test users with different morphologies.2
Safety
As with any mechanical wheelchair, a potential safety concern with the Gyrolift is the possibility of the device malfunctioning during use. This could put users in danger and compromise their safety and independence if they are injured.
Issues to Consider
While the Canadian price still unknown, the Gyrolift may be more expensive than other standing wheelchairs or electric wheelchairs and it is unclear whether potential users will have to pay out of pocket. For example, in Ontario, the current provincial health care plan does not cover standing wheelchairs and pays 75% of the cost for other mobility aids, with the remaining 25% paid out of pocket by the individual (unless they are receiving financial support from a subsidy program).4 Therefore, the Gyrolift may be available to those who can afford to pay out of pocket or who have additional insurance to cover its cost.
Related Developments
The Gyrolift is currently being piloted for use by individuals who are paraplegic, and the technology may be available in the future for others with limited mobility. Other stand-up robotic wheelchairs5,6 are currently on the market but do not have the same features as the Gyrolift wheelchair (such as the two-wheel Segway technology).
Looking Ahead
Results from future studies testing the maneuverability and safety of the Gyrolift, not only for individuals with paraplegia but also for those with other mobility concerns, will help to inform the best use of this technology in the future; however, there is no indication when this technology might be available for use in Canada.
References
- Gyrolift. Gyrolift: new mobility solution. 2019; http://www.gyrolift.fr/. Accessed 2019 Dec 12.
- Trenoras L, Gregory U, Monacelli E, Hugel V. Mechatronic design of the Gyrolift verticalization wheelchair. Paper presented at: 2014 IEEE/ASME International Conference on Advanced Intelligent Mechatronics; 2014 Jul 8-11; Besacon, France. 2014; https://ieeexplore.ieee.org/document/6878263/citations?tabFilter=papers#citations. Accessed 2019 Dec 4.
- Giesbrecht EM, Smith EM, Mortenson WB, Miller WC. Health reports: needs for mobility devices, home modifications and personal assistance among Canadians with disabilities [Catalogue no. 82-003-X]. Ottawa (ON): Statistics Canada; 2017 Aug 16: https://www150.statcan.gc.ca/n1/en/pub/82-003-x/2017008/article/54852-eng.pdf?st=d0BEWY3i. Accessed 2019 Nov 19.
- Ontario Ministry of Health and Long-Term Care. Mobility aids. 2020; https://www.ontario.ca/page/mobility-aids. Accessed 2020 Jan 9.
- UPnRIDE Robotics Ltd. Far more than a wheelchair: safety mobility outdoors and indoors while standing or sitting. 2019; https://upnride.com/. Accessed 2019 Dec 12.
- Matia Robotics (US) Inc. The Tek Robotic Mobilization Device (RMD). 2019; https://www.matiarobotics.com/. Accessed 2019 Dec 12.
Positron Emission Tomography-Computed Tomography Biology-Guided Radiotherapy for Cancer Treatment
Biology-guided radiotherapy (BgRT) combines the functional imaging data from positron emission tomography (PET) and the anatomic data from computed tomography (CT) into a single machine that guides personalized radiotherapy.1 Currently in development, RefleXion X1 is a hybrid PET-CT linear accelerator designed to one day enable real-time tracking of tumours, which would allow for better targeting of tumour movement and the treatment of multiple tumours in one session.1,2
How It Works
It is estimated that one in two Canadians will be diagnosed with cancer during their lifetime.3 More than half of patients with cancer will have radiotherapy as part of their treatment plan, either as monotherapy or with other modalities such as surgery and/or chemotherapy.4 As radiotherapy is typically delivered in multiple treatment fractions spread over days or weeks, image-guided radiotherapy using traditional anatomical imaging technologies such as X-ray, ultrasound, or magnetic resonance (MR) can help increase patient set-up accuracy, monitor internal organ motion, and minimize toxicity to at-risk surrounding organs.5
In contrast to anatomical imaging techniques, molecular image guidance using combined PET-CT provides both anatomic and metabolic information.6 With BgRT, a patient receives an intravenous injection of a radiotracer such as fluorine-18 fluorodeoxyglucose (a glucose analogue).1 As this radiotracer is similar in structure to glucose, it accumulates in areas of elevated sugar metabolism (such as in tumours).6 As the radioisotope breaks down, resulting in positron emissions, gamma ray photons are created.2 These emissions continuously broadcast the tumour’s location in real time, and can be detected by two PET arcs on the BgRT machine.1
As the first BgRT to use the actual tumour to guide radiotherapy,7 RefleXion X1’s clinical workflow starts with a traditional planning CT scan, and clinician contouring of tumour volumes and at-risk organs.1 After an imaging-only session on the RefleXion X1 machine, where a planning PET scan is obtained, a personalized treatment plan is created.1 At each subsequent treatment session, after confirmation with a brief pre-treatment PET scan, radiotherapy can be delivered.1 Throughout the radiation delivery process, the RefleXion X1 machine continuously gathers PET emissions, which are translated into beam delivery instructions to treat various cancer types.1
Who Might Benefit?
Due to smaller treatment margins and better tissue sparing compared with CT-based image guidance, BgRT has the potential to treat oligometastatic tumours in various disease sites such as lymph nodes, lung, breast, prostate, head, and neck.2 Due to its ability to track motion,1 RefleXion X1 can also be used to treat tumours that are subject to movement.8
Availability in Canada
RefleXion X1 is not currently available for sale in any market (Marketing Department, RefleXion Medical, Hayward, CA: personal communication, Jan 14, 2020). The company’s initial focus will be commercialization in the US, and subsequently in other markets (Marketing Department, RefleXion Medical: personal communication, Jan 14, 2020). At this time, the RefleXion machine is pending its first 510(k) clearance from the US FDA (Marketing Department, RefleXion Medical: personal communication, Jan 14, 2020).
What Does It Cost?
Although pricing information for RefleXion X1 is currently unavailable, its future list price will align with other premium radiotherapy machines (excluding proton beam therapy machines) (Marketing Department, RefleXion Medical: personal communication, Nov 25, 2019).
Current Practice
In the delivery of radiotherapy, CT scanning is the standard imaging modality for treatment simulation, tissue contouring, treatment planning, and image guidance.9 To facilitate dose escalation to tumours and tissue sparing to healthy organs, CT imaging before each treatment fraction allows for more localized treatments and helps minimize the risk of missing the tumour.10 Due to limitations of CT imaging such as motion-related artifacts and poor soft tissue contrast,10 other technologies are being considered for image guidance.
Currently, PET-CT is used in cancer diagnosis, staging, treatment planning, and response monitoring.11 In treatment planning and response monitoring, PET-CT is considered more accurate than CT only, and can be used in the following instances:11
- tumours that are difficult to visualize with CT or MR imaging (e.g., cancer that has metastasized to sites that are distant from the original tumour)
- treatment avoidance of tissues with non-tumour cells (e.g., collapsed lung tissue in patients with lung cancer)
- evaluating tumour response to concurrent chemoradiation.
What Is the Evidence?
As RefleXion X1 is awaiting clearance from the FDA,1 no published clinical trials were identified. Most identified studies pertain to treatment feasibility or dosimetry planning for PET-guided radiotherapy. In a proof-of-concept lung tumour tracking study, a phantom with spherical targets was designed to mimic respiratory motion and allow for the measurement of PET-guided target tracking accuracy.12 With the implementation of a dynamic tracking algorithm, tumour tracking error did not exceed a 2 mm threshold, and PET-guided tumour tracking was deemed to be clinically accurate.12
Simulated dose delivery studies involving digital patients with programmed 3-D motion have shown PET-guided radiotherapy to be capable of dose escalation to gross tumour volumes.13,14 With increased target accuracy, a radiation dose can be safely escalated while minimizing collateral damage to the surrounding healthy tissue, as shown by a simulation breast cancer study.14 In an ongoing clinical trial, people with early-stage lung cancer are being recruited to evaluate the planning feasibility of PET-guided radiotherapy.15 Specifically, the primary objective of the trial is to measure the variation in metabolic tumour volumes throughout a five-fraction treatment plan.15
Safety
To minimize radiation exposure to patients, health care providers, and the general public, radiation safety principles need to be followed during PET-CT scans due to the use of radioisotopes such as fluorine-18 fluorodeoxyglucose and the low radiation dose from the CT scan.16 On average, the general public naturally receives about 3 mSv of background radiation annually.17 A typical PET-CT scan gives off about 25 mSv of radiation.17 Thus, to align with the ALARA (i.e., as low as reasonably achievable) safety principle,16 RefleXion X1 BgRT could be appropriate for hypofractionated radiotherapy, where fewer treatment sessions are given.1
Immediately after the injection of a radiotracer, the patient is escorted to a secluded area to wait for 60 minutes before their PET-CT scan.16 As some radioactive material remain for a few hours after the scan, the patient should limit their contact with those who are most vulnerable to radiation (i.e., young children and pregnant people).17 Despite the use of fluorine-18 fluorodeoxyglucose, PET-CT scans are considered safe for patients with diabetes.18 Additionally, the annual occupation exposure for radiotherapy technologists setting up patients for PET-CT scans and radiation treatments would still fall under maximum allowable levels, assuming they are involved in one set-up per day.19
Issues to Consider
In addition to meeting radiation safety regulations, the implementation and use of BgRT in a cancer treatment centre would require consideration of implications to workflow,1 scheduling,19 and training.20 For cancer clinics without existing PET-CT scanners and nuclear medicine technologists, the radiation oncology team would have to coordinate closely with an external nuclear medicine team to prepare patients for their BgRT.1 In light of annual effective dose limits for nuclear energy workers, and lower limits for pregnant workers, careful consideration would have to be given in the scheduling of technologist staff.19,21 To limit the radiation exposure to staff during patient set-up, and to ensure timely and accurate image registration before radiation delivery, technologist staff would likely benefit from additional training.19,20
Related Developments
MR-guided linear accelerators have been developed and are in research stages at cancer centres in Edmonton and Toronto.22 With improved soft tissue contrast and the ability to image and treat in real time, MR-guided radiotherapy is likely suitable for tumour sites such as brain, liver, kidney, and lung, where lesions may not be well visualized with CT-based image-guidance techniques and/or where real-time motion tracking is critical to ensure treatment precision.9,10 Furthermore, in contrast with CT-based image guidance, MR-guided radiotherapy does not result in additional radiation exposure for those who receive the treatment.22
Due to its narrow range of dose deposition, particle therapy (such as proton therapy) can achieve superior sparing of surrounding healthy tissue when compared with photon therapy.23 To capitalize on this benefit and prevent missing the target, PET-guided particle therapy is being developed for its advantages in treatment monitoring and motion management.20
Looking Ahead
In addition to fluorine-18 fluorodeoxyglucose, the use of other radiotracers that allow for imaging tumour proliferation can be examined and used in the future for BgRT.1,20 A novel PET-CT radiotracer (68Ga-FAPI) has been recently identified to exhibit high tumour uptake and image quality, which may make it suitable for cancers such as sarcoma and esophageal cancer, where fluorine-18 fluorodeoxyglucose uptake is low.24
As a hybrid PET-CT linear accelerator designed to treat multiple lesions in one session and to better control for tumour motion, clinical trials are required to establish the efficacy of RefleXion X1.
Author: Yan Li
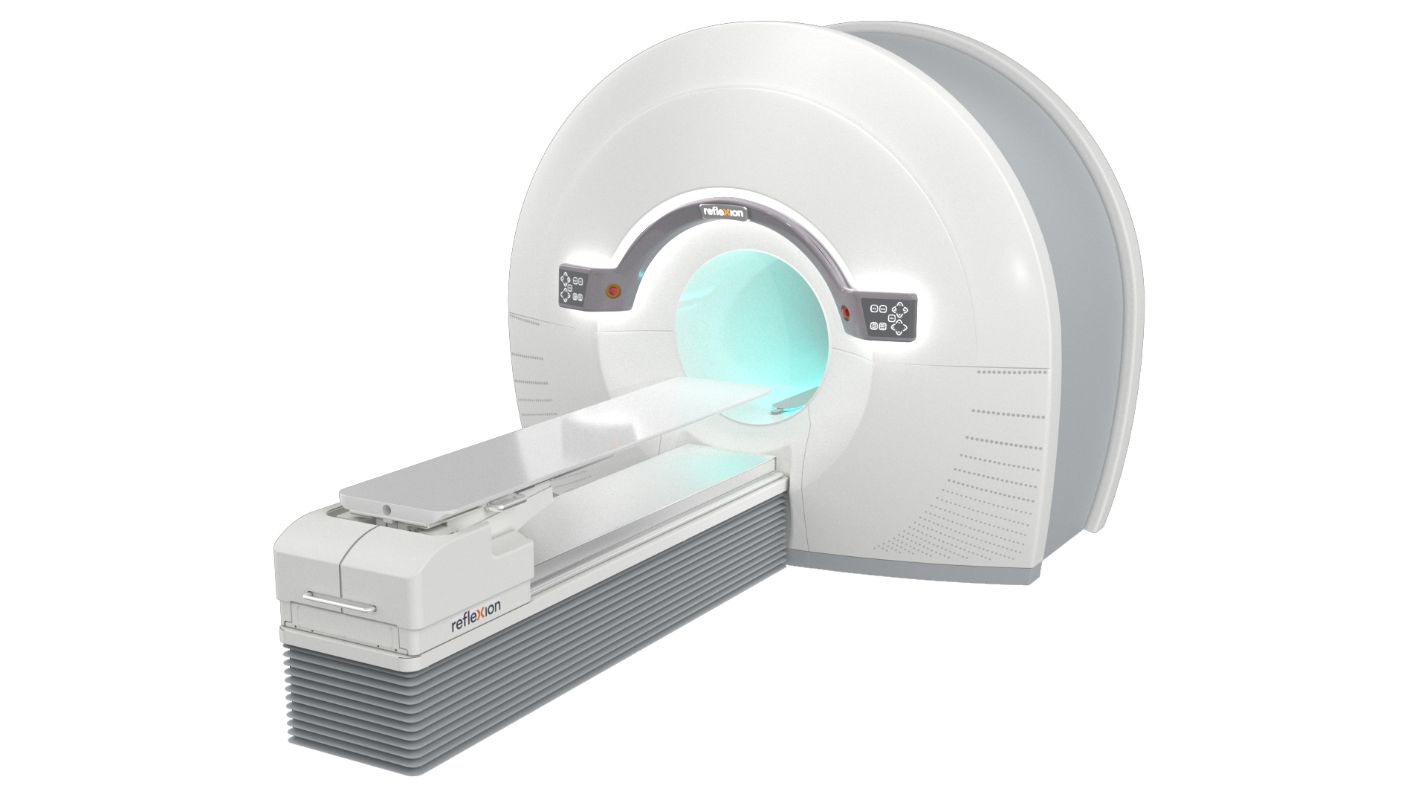
Image reproduced with permission from RefleXion Medical.
References
- Da Silva A, Mazin S. Treatment Planning and Delivery Overview of Biology-guided Radiotherapy. White paper. Hayward (CA): RefleXion: https://reflexion.com/wp-content/uploads/2019/10/BgRT_WhitePaper_Final.pdf. Accessed 2019 Nov 27
- Hwang M, Lalonde R, Heron D, Huq M. A clinical workflow for a prototype biology-guided radiation therapy (BGRT) machine. Med Phys. 2019;46 (6):e320.
- Canadian Cancer Society. Cancer statistics at a glance. Toronto: Canadian Cancer Society; 2020: https://www.cancer.ca/en/cancer-information/cancer-101/cancer-statistics-at-a-glance/?region=on. Accessed 2019 Nov 27.
- American Cancer Society. Radiation Therapy Basics. Atlanta (GA): American Cancer Society; 2018: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/radiation/basics.html. Accessed 2019 Nov 27.
- Sun B, Chang J, Rong Y. The more IGRT systems, the merrier? J appl clin med phys. 2017;18(4):7-11.
- Farwell MD, Pryma DA, Mankoff DA. PET/CT imaging in cancer: current applications and future directions. Cancer. 2014;120(22):3433-3445.
- RefleXion Medical. RefleXion to Unveil New Approach to Cancer Treatment at ASTRO. 2018 Oct 18; https://www.globenewswire.com/news-release/2018/10/18/1623451/0/en/RefleXion-to-Unveil-New-Approach-to-Cancer-Treatment-at-ASTRO.html. Accessed Nov 27, 2019.
- Casey B. RefleXion debuts PET-guided radiation therapy. 2018; https://physicsworld.com/a/reflexion-debuts-pet-guided-radiation-therapy/. Accessed 2019 Dec 6.
- Cao Y, Tseng C-L, Balter JM, Teng F, Parmar HA, Sahgal A. MR-guided radiation therapy: transformative technology and its role in the central nervous system. Neuro-Oncology. 2017;19(suppl_2):ii16-ii29.
- Mittauer K, Paliwal B, Hill P, et al. A New Era of Image Guidance with Magnetic Resonance-guided Radiation Therapy for Abdominal and Thoracic Malignancies. Cureus. 2018;10(4):e2422.
- Jelercic S, Rajer M. The role of PET-CT in radiotherapy planning of solid tumours. Radiology and oncology. 2015;49(1):1-9.
- Yang J, Yamamoto T, Mazin SR, Graves EE, Keall PJ. The potential of positron emission tomography for intratreatment dynamic lung tumor tracking: a phantom study. Med Phys. 2014;41(2):021718.
- Fan Q, Nanduri A, Mazin S, Zhu L. Emission guided radiation therapy for lung and prostate cancers: a feasibility study on a digital patient. Med Phys. 2012;39(11):7140-7152.
- Fan Q, Nanduri A, Yang J, et al. Toward a planning scheme for emission guided radiation therapy (EGRT): FDG based tumor tracking in a metastatic breast cancer patient. Med Phys. 2013;40(8):081708.
- Higgins K, Emory University. Fludeoxyglucose F-18-PET in Planning Lung Cancer Radiation Therapy. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine; 2018 Apr 11; updated 2019 Apr 25: https://clinicaltrials.gov/ct2/show/NCT03493789. Accessed 2019 Dec 6.
- Anderson J, Mathews D. Site Planning and Radiation Safety in the PET Facility. Alexandria (VA): American Association of Physicists in Medicine; 2002: https://www.aapm.org/meetings/02AM/pdf/8418-39272.pdf. Accessed 2019 Dec 9.
- American Cancer Society. Understanding Radiation Risk from Imaging Tests. 2018; https://www.cancer.org/treatment/understanding-your-diagnosis/tests/understanding-radiation-risk-from-imaging-tests.html. Accessed 2019 Dec 9.
- International Atomic Energy Agency. Radiation protection of patients during PET/CT scanning. https://www.iaea.org/resources/rpop/health-professionals/nuclear-medicine/pet-ct/patients. Accessed 2019 Dec 9.
- Sam S, Shon IH, Vinod SK, Lin P, Lin M. Workflow and radiation safety implications of (18)F-FDG PET/CT scans for radiotherapy planning. J Nucl Med Technol. 2012;40(3):175-177.
- Keall P, Kron T, Zaidi H. In the future, emission-guided radiation therapy will play a critical role in clinical radiation oncology. Med Phys. 2019;46(4):1519-1522.
- Canadian Nuclear Safety Commission. Radiation doses. 2019; http://nuclearsafety.gc.ca/eng/resources/radiation/introduction-to-radiation/radiation-doses.cfm. Accessed 2019 Dec 10.
- Morrison A. MR-linac for Radiation Therapy for the Treatment of Cancer. Health technology update no.23. Ottawa: CADTH; 2019: https://cadth.ca/sites/default/files/hs-eh/EN0013-health-technology-update-march-2019.pdf. Accessed 2019 Dec 6.
- Hu M, Jiang L, Cui X, Zhang J, Yu J. Proton beam therapy for cancer in the era of precision medicine. J Hematol Oncol. 2018;11(1):136-136.
- Kratochwil C, Flechsig P, Lindner T, et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med. 2019;60(6):801-805.
A New Point-of-Care Device for Clozapine Monitoring
The CSAN Pronto is a point-of-care device that aims to help ease some of the blood testing requirements associated with the use of clozapine for treatment-resistant schizophrenia.
How It Works
The point-of-care (PoC) Clozaril Support and Assistance Network (CSAN) Pronto diagnostic device is intended to be used to measure the white blood cell counts (WBC) and neutrophil percentages of those who have been prescribed clozapine for treatment-resistant schizophrenia (Dominique Gilbert, Director, Market Access and Health Policy, HLS Therapeutics Inc., Dorval, Que: personal communication, Dec 3, 2019). Typically, this blood testing is done by IV blood draw in a doctor’s office. The CSAN Pronto is a table-top device that is able to provide WBC counts and neutrophil percentages using a drop of blood (3.5 μL) from a finger prick instead of requiring an IV draw.1 The blood drop is directly applied onto a test strip that is then analyzed by the device. The results are sent to the CSAN system, where the results are available to the user and their physician immediately.1,2 The existing CSAN portal — an online resource and registry for people who have been prescribed Clozaril (clozapine) — was created as part of the Health Canada requirements for blood testing with the use of clozapine. The CSAN Pronto device will be integrated into the existing CSAN portal as an alternative way for patients to have their blood tested and to submit those results into the system (Dominique Gilbert: personal communication, Dec 3, 2019). The CSAN Pronto device will be available for hematological testing results related to the use of clozapine and, through the portal, can provide results of participants to the treating physician or pharmacist immediately.3
Who Might Benefit?
The CSAN Pronto device is intended to be used by adults diagnosed with treatment-resistant schizophrenia who have been prescribed clozapine as a part of their treatment. Worldwide, it is estimated that schizophrenia affects 1% of the population.4 That number translates to roughly 370,000 Canadians. Approximately 25% to 30% of people diagnosed with schizophrenia meet the criteria for treatment-resistant disease.5
In 1975, clozapine was removed from the Canadian market due to reports of life-threatening side effects associated with agranulocytosis (i.e., a low number of white blood cells).6 Clozapine was made available to Canadians again in 1991 under the condition that everyone prescribed clozapine be enrolled in a patient registry to monitor adverse events.6 In addition, to combat agranulocytosis, white blood cell levels must be measured before each prescription refill can be issued.6 This means people taking clozapine for treatment-resistant schizophrenia are required to test their WBC weekly for at least the first six months of treatment.3 Nonadherence to blood work requirements has been cited by psychiatrists as one of the major barriers to treatment with clozapine.1,4
Availability in Canada
The CSAN Pronto was granted a medical device licence by Health Canada on October 29, 2019 (Dominique Gilbert: personal communication, Dec 3, 2019). HLS Therapeutics Inc. is working toward setting up the commercial program and anticipates the CSAN Pronto will be available to Canadians in the first quarter of 2020 (Dominique Gilbert: personal communication, Dec 3, 2019). The CSAN Pronto device will only be available and accessible through the CSAN program to those who have been prescribed clozapine for treatment-resistant schizophrenia (Dominique Gilbert: personal communication, Dec 3, 2019).
What Does It Cost?
CSAN Pronto will not be available for purchase to those who are not a part of the CSAN program (Dominique Gilbert: personal communication, Dec 3, 2019). It is unclear whether the use of this device presents an additional cost to the user. No information regarding the costs associated with the use of the device, or the required test strips, was available.
Current Practice
Treatment resistance in schizophrenia is most commonly defined as non-response to trials of two different antipsychotic medications.5 Canadian practice guidelines recommend clozapine as the only practical treatment option for people diagnosed with treatment-resistant schizophrenia.5As previously stated, all people receiving clozapine must be enrolled in a patient registry to facilitate the monitoring of their WBC counts before receiving a prescription refill because of the potential for agranulocytosis.6 Currently, recipients of clozapine must have a blood test done weekly for the first 26 weeks of treatment and then continue to have their WBC monitored bi-weekly for next 26 weeks, every four weeks after one year.3 Weekly testing is reinstated for a period of six weeks if treatment is missed for more than three days.3 Monitoring of the WBC must also continue weekly for at least four weeks after treatment with clozapine has been stopped.3 Considering the risk of adverse effects associated with clozapine, guidelines recommend that anyone not responding to treatment with clozapine stop taking the drug.3
What Is the Evidence?
No published studies were found that reported on the use of the CSAN Pronto by people taking clozapine. One validation study undertaken in people with cancer was identified for the US version of the device (Athelas One).7 The authors reported that the Athelas One device provided similar WBC and neutrophil counts when compared with venous blood samples and traditional laboratory testing.7
Safety
No specific evidence relating to the safety of the CSAN Pronto device was identified nor were any safety issues mentioned in the literature that was reviewed.
Issues to Consider
Schizophrenia is a complex psychiatric disorder that currently does not have a cure and requires treatment with antipsychotic drugs for the duration of a person’s life.4 People with schizophrenia often experience other medical, psychiatric, or substance use disorders at the same time and may experience instability with respect to food, income, clothing, and housing.8
There is a discrepancy between the number of people eligible to take clozapine and the number of prescriptions dispensed. It is thought that this discrepancy is partly due to the barriers and burdens associated with receiving this drug.4 As mentioned previously, the frequency of blood monitoring is a known barrier to clozapine treatment. It may be that providing people with an easier and more convenient way to fulfill the testing requirement could improve uptake and compliance.
Related Developments
Other PoC devices are available for use globally that measure blood component levels for both clozapine monitoring and other hematological monitoring requirements.1,9,10 Additionally, PoC assay devices are available that analyze clozapine levels in a person’s blood using a finger prick.11
In the US, the CSAN Pronto is available under a different brand name (Athelas One)2 and it has been cleared by the FDA for WBC and neutrophil count testing.2
Looking Ahead
Improvements in health outcomes have been found to be greater when people have more access to their own health information and are engaged with their health.12 While not everyone is immediately comfortable with the addition of personal technologies to health care, as more health-related apps and PoC tests enter the market, their use will likely become a more standard and accepted part of how people’s health is managed.
Michelle Clark
References
- Kalaria SN, Kelly DL. Development of point-of-care testing devices to improve clozapine prescribing habits and patient outcomes. Neuropsychiatr Dis Treat. 2019;15:2365-2370. Athelas. Athelas [manufacturer website]. 2020; https://athelas.com/. Accessed 2020 Jan 15.
- HLS Therapeutics Inc. Product Monograph: Clozaril. 2019; http://www.hlstherapeutics.com/wp-content/uploads/2016/04/HLS-Clozaril-PM-E.pdf. Accessed 2020 Jan 13.
- Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses. 2018;12(1):23-30.
- Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry. 2017;62(9):604-616.
- Health Canada. Summary Safety Review - Clozapine - Assessing the effectiveness of monitoring for low numbers of white blood cells. 2018; https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/clozapine-white-blood-cells.html. Accessed 2020 Jan 13.
- Dale DC, Kelley ML, La Vega MND, et al. A novel device suitable for home monitoring of white blood cell and neutrophil counts. Blood. 2018;132(Suppl 1):1103.
- Schizophrenia Society of Canada. Learn more about schizophrenia. 2020; https://www.schizophrenia.ca/learn_more_about_schizophrenia.php. Accessed 2020 Jan 20.
- Bui HN, Bogers JP, Cohen D, Njo T, Herruer MH. Evaluation of the performance of a point-of-care method for total and differential white blood cell count in clozapine users. Int J Lab Hematol. 2016;38(6):703-709.
- Van Pelt H, Chevallier M, Wilbie W, Schoorl M. Evaluation of three POCT hematology analyzers for white blood cell analysis. Int J Lab Hematol. 2016;38(Suppl 2):112.
- Sumanth A, Kozo D, Courtney J, et al. Novel point of care testing device for rapid measurement of clozapine in whole blood. Clin Chim Acta. 2019;493(Suppl 1):S658-S659.
- Canada Health Infoway. Annual report 2018-2019: a new day in health care is coming. 2019; https://www.infoway-inforoute.ca/en/component/edocman/3726-annual-report-2018-2019/view-document?Itemid=101. Accessed 2020 Jan 15.

ER-REBOA: An Emergency Intervention for Non-Compressible Abdominal Bleeding
Originally developed by trauma surgeons in a military-civilian collaboration, resuscitative endovascular balloon occlusion of the aorta, or REBOA, is a technique that controls non-compressible bleeding in patients with abdominal trauma.1 As non-compressible bleeding of the torso is a major cause of potentially preventable death, Prytime Medical ER-REBOA device serves as an emergency intervention to avert irreversible hemorrhagic shock and subsequent cardiorespiratory arrest. 2
How it Works
In general, the REBOA technique is a temporary, minimally invasive measure used to control abdominal bleeding and sustain blood flow to the heart and brain until a more definitive intervention can be performed.1 Patients with abdominal injury who are hypotensive and not responsive to initial blood product resuscitation would be suitable candidates for this procedure.1 Chest X-rays can be used to rule out contraindications, such as thoracic vascular injury or aortic injury.1
The ER-REBOA device is the first balloon occlusion catheter to be designed and marketed specifically for the fields of trauma, critical care, and emergency medicine.3 First, with the assistance of ultrasound, an arterial sheath is inserted to access the blood vessel, most commonly through the common femoral artery.1 Through this sheath, the ER-REBOA catheter and balloon are positioned at the appropriate anatomical zone of the aorta.1 The balloon is inflated, which temporarily stops abdominal bleeding.1 The ER-REBOA components are then deflated and removed after patients are transferred to the operating room, ruptured vessels are then mechanically occluded for more permanent hemorrhage control.1
Who Might Benefit?
In some cases of abdominal hemorrhage, direct pressure or a tourniquet can’t be used to control bleeding in certain non-compressible areas of the torso: these include the pulmonary system, axial vessels, and solid organs.4 An intervention like REBOA can prevent fatality from non-compressible bleeding of the torso.2 Once the artery is successfully accessed, REBOA can be a faster procedure than the current practice of resuscitative thoracotomy, which makes it beneficial in emergency situations.1 REBOA can be used in patients who opt out of blood transfusions.1 Patients in areas with a limited availability of blood products can also benefit from the REBOA technology.1
Availability in Canada
The ER-REBOA catheter and Convenience Kit have been licensed by Health Canada since 2017 and 2018, respectively.5,6
What Does it Cost?
The cost of Prytime Medical’s ER-REBOA catheter in Canada is currently unavailable.
A one-year cost-utility analysis compared REBOA to the current practice of resuscitative thoracotomy with aortic cross-clamping for the treatment of non-compressible torso hemorrhage.7 Using costing guidelines from the UK’s National Health Service, the authors found that REBOA was less cost-effective.7
Current Practice
Standard practice for patients with non-compressible hemorrhage below the diaphragm is resuscitative thoracotomy with aortic occlusion via open cross-clamp.1
What is the Evidence?
Authors of a systematic review comprising of studies that used various REBOA devices, including ER-REBOA, reported results of 1,340 patients from several case series and cohort studies and noted the lack of clear evidence supporting the use of the REBOA technique for improving mortality outcomes.2
The Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery Registry by the American Association for the Surgery of Trauma contains prospective from trauma patients who have undergone various interventions, including REBOA.8 The REBOA technique was found to have similar procedure time and mortality outcomes when compared to open aortic occlusion, with minimal complications.8
Two ongoing studies will further elucidate the clinical and economic outcomes of the ER-REBOA device and the technique in general. The Emergent Truncal Hemorrhage Observational Study, funded by the US Department of Defense, will compare various REBOA devices, including the ER-REBOA catheter, to other abdominal hemorrhage control procedures in patients admitted to trauma centres.9 Additionally, the first randomized controlled trial to compare standard major trauma centre treatment plus the REBOA technique to standard care alone is currently taking place in the UK.10
Safety
While the risk of REBOA complications has been found to be low at 6.4%, complications include pseudoaneurysm, embolism, and limb ischemia.8 They can result from over-inflation, prolonged inflation, or rapid deflation of the REBOA balloon, emphasizing the importance of training.1The risk of complications rises with the increasing size of the arterial sheath that is used to guide the catheter.1
Issues to Consider
A variety of implementation issues could limit REBOA utilization. Immediate surgical intervention for definitive hemorrhage control is needed following REBOA. In a case series of 109 patients in Japan, both elapsed time from hospital arrival to initial arterial access and duration of occlusion were shorter among survivors.11,12 Accessibility of surgical facilities should be considered before the use of REBOA. ER-REBOA would likely be best implemented in scenarios where patients are quickly transferred to a facility with emergency responders and clinicians who are familiar with the intervention and are able to provide surgical hemorrhage control.1
Rural and remote pre-hospital settings would not be ideal for the application of REBOA. Confirming proper positioning of the catheter before inflating the balloon could be challenging in the absence of appropriate imaging devices.1 A lack of surgeons with vascular expertise to perform the procedure could be an additional barrier to implementation.1
Related Developments
The REBOA technique has possible indications for non-traumatic hemorrhage. REBOA has been used in elective C-sections where there is atypical placental implantation, as it reduces the large volume blood transfusions that would otherwise be necessary in these high-risk deliveries.1 Considering that hemorrhage is the most prevalent direct obstetric cause of death, the application of REBOA in managing peri- and post-partum hemorrhage could have an impact on outcomes.13
An early feasibility trial by Yale University is currently recruiting patients to assess the safety and efficacy of the ER-REBOA catheter in increasing blood perfusion to the brain following cardiac arrest.14
Looking Ahead
Further development of the partial REBOA technique, where the balloon is not completely inflated to the point of aortic occlusion, could mitigate current implementation issues.1 Partial occlusion allows for the balloon to remain inflated for longer, thus potentially increasing the window of time before patients must receive subsequent surgical hemorrhage control.1Large multi-centre trials are needed to draw definite conclusions on whether the ER-REBOA device results in better clinical outcomes compared to standard care.
By: Diksha Kumar
References
- Osborn LA, Brenner ML, Prater SJ, Moore LJ. Resuscitative endovascular balloon occlusion of the aorta: current evidence. Open Access Emerg Med. 2019;11:29-38.
- Petrone P, Perez-Jimenez A, Rodriguez-Perdomo M, Brathwaite CEM, Joseph DK. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the management of trauma patients: a systematic literature review. Am Surg. 2019;85(6):654-662.
- Prytime Medical. Pryor Medical Devices receives FDA 510(k) clearance for distribution of its ER-REBOA catheter. 2015; http://prytimemedical.com/wp-content/uploads/2017/05/pressrelease_pmd-510k-approval-press-release-final.pdf. Accessed 2020 May 28.
- Long B, Hafen L, Koyfman A, Gottlieb M. Resuscitative endovascular balloon occlusion of the aorta: a review for emergency clinicians. J Emerg Med. 2019;56(6):687-697.
- ER-REBOA catheter; licence no. 99868. Medical devices active licence listing (MDALL) 2017; https://health-products.canada.ca/mdall-limh/index-eng.jsp. Accessed 2020 May 15.
- ER-REBOA catheter convenience set; licence no. 101467. Medical devices active licence listing (MDALL) 2018; https://health-products.canada.ca/mdall-limh/index-eng.jsp. Accessed 2020 May 15.
- Renna MS, van Zeller C, Abu-Hijleh F, Tong C, Gambini J, Ma M. A one-year cost-utility analysis of REBOA versus RTACC for non-compressible torso haemorrhage. Trauma. 2019;21(1):45-54.
- DuBose JJ, Scalea TM, Brenner M, et al. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409-419.
- Prytime Medical. Open clinical trials/registries. 2019; https://prytimemedical.com/news/active-clinical-studies/. Accessed 2020 May 15.
- University of Aberdeen. The UK-REBOA (Resuscitative Endovascular Balloon Occlusion of the Aorta) trial. 2017; https://w3.abdn.ac.uk/hsru/REBOA/Public/Public/index.cshtml. Accessed 2020 May 28.
- Matsumura Y, Matsumoto J, Kondo H, et al. Early arterial access for resuscitative endovascular balloon occlusion of the aorta is related to survival outcome in trauma. J Trauma Acute Care Surg. 2018;85(3):507-511.
- Reva VA, Matsumura Y, Horer T, et al. Resuscitative endovascular balloon occlusion of the aorta: what is the optimum occlusion time in an ovine model of hemorrhagic shock? Eur J Trauma Emerg Surg. 2018;44(4):511-518.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323-333.
- Yale University. NCT03703453: Resuscitative EndoVascular Aortic Occlusion for Maximal Perfusion (REVAMP). ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018: https://clinicaltrials.gov/ct2/show/NCT03703453. Accessed 2020 May 28.
The Optimizer Smart System: A New Device to Manage Chronic Heart Failure
Chronic heart failure is a progressive disease characterized by a variety of ventricular or valvular dysfunctions and is often initially treated with a cocktail of medications.1 The Optimizer Smart system provides an option for the treatment of moderate-to-severe chronic heart failure patients who are symptomatic despite optimal drug therapy and who are not candidates for cardiac resynchronization therapy (CRT).2
How It Works
The Optimizer Smart system is a programmable device used for cardiac contractility modulation therapy in patients with chronic heart failure.2 The system is comprised of an implantable pulse generator, pacing leads, and a mini-charger.3 The pulse generator is subcutaneously implanted into the right pectoral region of the chest and connected to two ventricular leads.3 The device sends a high voltage electrical impulse to the right ventricular septum during the absolute refractory period, which improves cardiac muscular contraction.4 By increasing the strength of the heart’s contractions, the device may improve symptoms of chronic heart failure such as six-minute walking distance, quality of life, and the functional status of patients.2
Who Might Benefit?
Chronic heart failure is an increasingly prevalent medical condition in Canada, with 50,000 newly diagnosed Canadians each year.5 In 2016, there were an estimated 600,000 Canadians living with chronic heart failure.5
The Optimizer Smart system is indicated for up to 70% of patients classified as having New York Heart Association (NYHA) Class III heart failure.2 It is intended for those who are 18 years or older, in sinus rhythm (i.e., are not indicated for CRT, QRS duration of less than 130 milliseconds), with a left ventricular ejection fraction (LVEF) of 25% to 45%, and who remain symptomatic despite optimal drug therapy.3 It is anticipated that the majority of patients eligible for treatment with the Optimizer Smart system will also have an implantable cardioverter-defibrillator (ICD).3
Availability in Canada
The Optimizer Smart system is not currently available in Canada.6 However, the Optimizer Smart system is available in numerous other countries including the US, the UK, Australia, and Germany.3
What Does It Cost?
The Canadian cost of the Optimizer Smart system is not currently available. The total implantation cost of the Optimizer Smart system in the UK is estimated to be £18,365.7 This cost is in addition to the estimated lifetime drug therapy for heart failure (standard of care) cost of £1,362.7 Furthermore, according to a model cited by the UK’s NICE‒National Institute for Health and Care Excellence, those who use the device and drug therapy gain 5.26 quality-adjusted life-years versus 4.0 quality-adjusted life-years in those who use drug therapy alone.7
Current Practice
The latest update of theCanadian Cardiovascular Society guidelines for the management of heart failure1 recommends that patients with a reduced ejection fraction (i.e., LVEF ≤ 40%) and symptoms of heart failure should be initiated on triple-drug therapy with an angiotensin-converting enzyme inhibitor, beta-blocker, and mineralocorticoid receptor antagonist. A diuretic may also be used, when needed, to maintain fluid balance.1 Various drug add-on therapies are also avaiIable for those who remain symptomatic despite initial triple-drug therapy.1 However, if patients continue to have symptoms and have a LVEF < 35% despite optimal drug therapy for at least three months, ICD and CRT (i.e., a pacemaker) device therapy may be warranted.1
What Is the Evidence?
Two multi-centre, randomized controlled trials8,9 both comparing cardiac contractibility modulation via the Optimizer Smart device plus optimal medical therapy versus optimal medical therapy alone were identified. One trial9 was conducted at 50 centres in the US (n = 428) and included participants with heart failure and NYHA Class III to Class IV symptoms, normal sinus rhythm, and an ejection fraction ≤ 35%. The other trial8 was conducted in the US, Germany, and Czechia — the Czech Republic — (n = 160) and included participants with heart failure with NYHA Class III to Class IV symptoms, normal sinus rhythm, and an ejection fraction of 25% to 45%.
Outcomes reported by the authors8,9 include:
- change in anaerobic threshold9
- change in peak VO2
- change in Minnesota Living with Heart Failure Questionnaire (MLHFQ)
- change in six-minute hall walk
- change in NYHA classification
- safety.
Furthermore, one systematic review with meta-analysis,4 which included the two randomized controlled trials previously mentioned, as well as two earlier randomized controlled trials, was identified. The systematic review4 reported all-cause mortality, total hospitalizations, worsening heart failure and heart failure-related hospitalizations, cardiac arrhythmias, ICD sensing defects and malfunction, six-minute walking distance, and MLHFQ. Overall, when the data from the four trials was combined, cardiac contractibility modulation significantly improved MLHFQ, but did not significantly improve all-cause mortality, worsening heart failure and heart failure-related hospitalizations, or six-minute walking distance.4
Safety
The identified studies reported potential safety issues related to device implantation including lead dislodgement,8,9 device pocket infections,9 and generator erosion.8 The manufacturer2 also lists the following as potential adverse effects occurring secondary to cardiac contractibility modulation signal delivery:
- abnormal cardiac function
- atrial and ventricular tachyarrhythmias
- atrial and ventricular bradyarrhythmias
- worsening heart failure
- myocardial tissue damage
- chest pain.
Related Developments
The Optimizer Smart system is currently being investigated for use in patients with preserved ejection fraction heart failure (i.e., LVEF ≥ 50%) with NYHA Class II or Class III symptoms.10 This pilot study is estimated to be completed by March 31, 2021.10
Looking Ahead
The incidence of cardiovascular disease is increasing with the aging Canadian population.11 Improvements to treatment methods also equates to people living longer with cardiovascular disease.5 As chronic heart failure is often the end result of cardiovascular disease, the occurrence of chronic heart failure is consequently increasing in Canada.5 This is leading to increased interest in new research in the treatment of chronic heart failure.5 Cardiac contractibility modulation is a novel method of treating patients with symptomatic heart failure.2 However, more evidence comparing the Optimizer Smart system to the standard of care is needed to ascertain the clinical benefit of cardiac contractibility modulation in improving the functional status and quality of life of patients with heart failure.
Author: Christopher Freige
References
- Ezekowitz JA, O'Meara E, McDonald MA, et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33(11):P1342-1433.
- Impulse Dynamics. 2020; https://impulse-dynamics.com/. Accessed 2020 Feb 7.
- PMA P180036: summary of safety and effectiveness data (SSED). Rockville (MD): U.S. Food & Drug Administration; 2019: https://www.accessdata.fda.gov/cdrh_docs/pdf18/P180036B.pdf. Accessed 2020 Feb 7.
- Mando R, Goel A, Habash F, et al. Outcomes of cardiac contractility modulation: a systematic review and meta-analysis of randomized clinical trials. Cardiovasc Ther. 2019;2019 (no pagination)(9769724).
- 2016 report on the health of Canadians: the burden of heart failure. Heart & Stroke Foundation; 2016: https://www.heartandstroke.ca/-/media/pdf-files/canada/2017-heart-month/heartandstroke-reportonhealth-2016.ashx?la=en&hash=91708486C1BC014E24AB4E719B47AEEB8C5EB93E. Accessed 2020 Feb 7.
- Government of Canada. Medical Devices Active Licence Listing (MDALL). 2019: https://health-products.canada.ca/mdall-limh/index-eng.jsp. Accessed 2020 Feb 7.
- National Institute for Health and Care Excellence. The OPTIMIZER smart system for managing heart failure. (Medtech innovation briefing MIB186) 2019: https://www.nice.org.uk/advice/mib186/resources/the-optimizer-smart-system-for-managing-heart-failure-pdf-2285963701388485. Accessed 2020 Feb 7.
- Abraham WT, Kuck KH, Goldsmith RL, et al. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail. 2018;6(10):874-883.
- Kadish A, Nademanee K, Volosin K, et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J. 2011;161(2):329-337.
- Impulse Dynamics. NCT03240237: CCM in heart failure with preserved ejection fraction (CCM-HFpEF). ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018: https://clinicaltrials.gov/ct2/show/NCT03240237?term=optimizer&draw=1&rank=7. Accessed 2020 Feb 7.
- Heart disease in Canada: highlights from the Canadian Chronic Disease Surveillance System. Ottawa (ON): Public Health Agency of Canada; 2017: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/heart-disease-canada-fact-sheet.html. Accessed 2020 Feb 7.
Venus P- Valve: A Self-Expanding Transcatheter Pulmonary Valve Replacement Device.
The Venus Pulmonary Valve (Venus P-Valve) is a self-expanding percutaneous pulmonary implantation device that offers an intervention to address right ventricle outflow tract (RVOT) complications stemming from tetralogy of fallot (ToF).1
How It Works
The purpose of the Venus P-Valve is to provide an intervention for RVOT enlargement. An RVOT enlargement is often the result of a pulmonary obstruction and can contribute to a leaky pulmonary valve that allows blood to flow back into the heart chamber.2 The Venus P-Valve is a stent consisting of a self-expanding valve and is delivered and implanted via a catheter to act as a valve replacement for the enlarged RVOT.1,3 The stent is anchored in the RVOT and is made of a fibre called nitinol.1 The design of the Venus P-Valve can accommodate RVOT diameters up to 32 mm to 33 mm, which is larger than other commercially available pulmonary valves.4 This procedure is commonly referred to as a percutaneous pulmonary valve implantation (PPVI).1,5,6
Who Might Benefit?
ToF accounts for 6.7% of all babies born with congenital heart disease and RVOT obstruction is a common heart abnormality associated with ToF.7,8 Patients with ToF typically undergo corrective surgery within the first six months of their lives; however, interventions in early childhood often lead to complications that require additional surgical correction later in life.7,8 Patients with right ventricle complications stemming from ToF can undergo PPVI as an alternative to repeated surgery.7,9 The commonly used PPVI devices are not suitable for large RVOT implantations, but the Venus P-Valve is designed specifically for dilated or enlarged RVOT resulting from surgical repair of ToF.2,9 Given that the Venus P-Valve is designed to accommodate larger RVOT diameters, it may provide treatment to patients who cannot receive the current commercially available PPVI devices.1,9
Availability in Canada
There is no indication that the Venus P-Valve is licensed in Canada. The Venus P-Valve has received approval from the Chinese FDA and has received Institutional Review Board approval for clinical testing;3 however, it has not been granted CE certification or US FDA approval.1,2,6 A CE feasibility and safety study is currently being conducted.2
What Does It Cost?
No information related to costs in Canada or elsewhere was identified.
Current Practice
The results of ToF repairment surgery are generally good, with approximately 95% of patients expected to survive to adulthood.2 In most cases, the ToF repairment surgery is associated with RVOT obstruction later in life and most centres now consider PPVI to be first-line therapy.2 The currently available PPVI devices (the Melody Valve or the Sapien Valve) are recommended for RVOT implantation, but not all patients who have undergone ToF repairment have RVOT dimensions that are compatible with the capacity of these devices.1,2
What is the Evidence?
Early procedural studies on the Venus P-Valve show safe and promising advances in PPVI. Six non-randomized studies were identified in the available literature.1,3-6,9 Five of these studies were prospective cohort studies that aimed to capture the early results regarding feasibility, effectiveness, safety, and short-term follow-up for Venus P-Valve implantations for appropriate candidates.1,3,5,6,9 One identified study used a retrospective approach to collect procedural and early-to-midterm follow-up information.4 Overall, the identified studies indicate general success related to valve implantation, encouraging health outcomes, and evidence of surgical reproducibility. The majority of studies had a follow-up of 12 months or less, and the sample sizes were from five to 55 patients.1,3-6,9 One study reported MRI follow-up data for a mean of 112 months.4 Two clinical trials were identified from the ClinicalTrials.gov database; one is in the recruitment phase10 and the status of the other is unknown.11
Safety
Of the identified studies that reported evidence related to safety for the Venus P-Valve, one study reported no severe adverse events,6` while another reported procedure complications related to sheath breakage and implantation migration and displacement.4 Additionally, one case report identified two cases of stent infolding of the Venus P-Valve after implantation.12
Issues to Consider
The Melody and Sapien devices, both currently available for smaller RVOT diameters, have reported complications related to stent fractures of the valve frame; conduit ruptures; coronary compression, which may prevent a PPVI intervention; and endocarditis or an infection associated with the PPVI procedure.7 It is possible that the Venus P-Valve could have similar complications.
Related Developments
The available literature identified two alternative devices similar to the Venus P-Valve. These devices include the Medtronic Harmony TPV and the Alterra Adaptive Prestent from Edwards Lifesciences.7 These devices were created to address the needs of patients with enlarged RVOTs who are not candidates for the currently recommended devices.7 The Harmony TPV device encompasses a larger valve diameter compared to the commercially available Melody Valve, similar to the Venus P-Valve.7 The Alterra Adaptive Prestent is “stent-like” and helps to reshape the RVOT13 by working similar to a docking station for the Sapien S3 valve.7
Looking Ahead
The Venus P-Valve may provide an additional valve option for patients with an enlarged RVOT who need a PPVI.9 The findings from the identified literature show reliable reproducibility and evidence of success in early cohort studies; however, more clinical trials with longer follow-up data and more reporting on patient-important outcomes are needed.2
Author: Shannon Hill
References
- Garay F, Pan X, Zhang YJ, Wang C, Springmuller D. Early experience with the Venus P-valve for percutaneous pulmonary valve implantation in native outflow tract. Neth Heart J. 2017;25(2):76-81.
- Qureshi SA, Jones MI. Recent advances in transcatheter management of pulmonary regurgitation after surgical repair of Tetralogy of Fallot [version 1; referees: 3 approved]. F1000Research. 1000;7.
- Cao QL, Kenny D, Zhou D, et al. Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2014;84(7):1131-1137.
- Morgan G, Prachasilchai P, Promphan W, et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve: international experience. EuroIntervention. 2019;14(13):1363-1370.
- Zhou D, Pan W, Jilaihawi H, et al. A self-expanding percutaneous valve for patients with pulmonary regurgitation and an enlarged native right ventricular outflow tract: one-year results. EuroIntervention. 2019;14(13):1371-1377.
- Husain J, Praichasilchai P, Gilbert Y, Qureshi SA, Morgan GJ. Early European experience with the Venus P-valve: filling the gap in percutaneous pulmonary valve implantation. EuroIntervention. 2016;12(5):e643-651.
- Balzer D. Pulmonary valve replacement for Tetralogy of Fallot. Methodist Debakey Cardiovasc J. 2019;15(2):122-132.
- Driesen BW, Warmerdam EG, Sieswerda GJ, et al. Percutaneous pulmonary valve implantation: current status and future perspectives. Curr Cardiol Rev. 2019;15(4):262-273.
- Promphan W, Prachasilchai P, Siripornpitak S, Qureshi SA, Layangool T. Percutaneous pulmonary valve implantation with the Venus P-valve: clinical experience and early results. Cardiol Young. 2016;26(4):698-710.
- Venus MedTech (HangZhou) Inc. NCT02846753: implantation of the Venus P-Valve™ in the pulmonic position in patients with native outflow tracts. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2016: https://clinicaltrials.gov/ct2/show/record/NCT02846753?term=Venus+P-Valve&draw=2&rank=2. Accessed 2020 Mar 6.
- Venus MedTech (HangZhou) Inc. NCT02071654: efficacy and safety evaluation of transcatheter pulmonary valve implantation for right ventricular outflow tract (RVOT) stenosis after congenital heart defect surgical correction complicated with severe pulmonary regurgitation. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2014: https://clinicaltrials.gov/ct2/show/record/NCT02071654?term=Venus+P-Valve&draw=2&rank=1. Accessed 2020 Mar 6.
- Riahi M, Ang HL, Jones M, et al. Infolding of the Venus P-Valve after transcatheter pulmonary valve implantation. Circ Cardiovasc Interv. 2018;11(4):e005923.
- Edwards Lifesciences’ Alterra adaptive prestent device used for first time to treat a malformed pulmonary valve. Cardiovascular News 2017; https://cardiovascularnews.com/edwards-lifesciences-alterra-adaptive-prestent-device-used-first-time-treat-malformed-pulmonary-valve/ Accessed 2020 Mar 19.
SoundBite: Using Shock Waves to Treat Chronic Total Occlusions
Chronic total occlusions (CTOs) in patients with coronary heart disease are associated with a higher risk of adverse events, a decrease in quality of life, and increased health care costs.1 Novel crossing technology by Soundbite Medical Solutions aims to break down arterial calcification using short-duration high-amplitude pressure pulses called shock waves.1
How It Works
Soundbite Medical Solutions’ Soundbite Crossing system consists of a disposable wire, guided by a supportive balloon catheter, that percutaneously penetrates the artery.1 The other component of the Soundbite system is a console that generates low-frequency, concentrated shock waves at the proximal end of the guide wire, which then mechanically propagate to the distal tip where the occlusion is located.1 Due to the shock waves, the distal tip then rapidly oscillates and crosses through arterial calcification.1 If the Soundbite system successfully creates a space within the CTO, a therapeutic system, such as a stent, can be inserted (if necessary).1
Current Practice
While pharmacological therapy can be sufficient for some patients with coronary heart disease, percutaneous coronary interventions (also called angioplasties) are the preferred treatment for revascularization in those with CTOs.2
Percutaneous coronary interventions consist of high-pressure balloon dilations, where an interventional cardiologist inserts a guide wire and subsequently a balloon catheter across the blockage site using fluoroscopy.3 The balloon at the tip of the catheter is then inflated and deflated continuously to push calcified plaque outward against the arterial wall, opening up the narrowed blood vessel.3 In the case of a total occlusion, new collateral blood vessels may have formed to redirect blood flow around the blockage.4 The catheter is also used to widen these collateral vessels.4
The challenges with this method of angioplasty include the risk of injuring non-calcified arterial segments when directing the guide wire, as well as the possibility of not producing enough force to fracture the calcium.5 Percutaneous coronary interventions are technically demanding, and failure to reach calcified lesions with a guide wire is the main reason for their lower success rate.1
CTOs can also be treated by a surgical procedure called coronary artery bypass grafting, which is generally reserved for patients with complex or multivessel disease.2 In this procedure, peripheral blood vessels, such as veins from the legs or arteries from the chest or arms, are used to connect to blood vessels above and below the coronary occlusion, thus redirecting blood flow.6
Who Might Benefit?
In a Canadian multi-centre registry, 18.4% of patients with coronary heart disease were found to have CTOs.7 Thus, among the 2.4 million Canadians diagnosed with heart disease,8 it is possible that 440,000 of them may require intervention to manage either coronary or peripheral CTOs. Patients who require a stent to treat their narrowed arteries could benefit from shock wave crossing to clear calcified occlusions before the insertion of a stent.9 Calcified plaques not only affect stent positioning and expansion, but also drug delivery.5
Availability in Canada
The Soundbite Crossing system is currently approved by Health Canada for the treatment of CTOs associated with peripheral artery disease, but not those associated with coronary artery disease.10 As of January 2020, Soundbite has also received 510(k) clearance by the FDA for use in the treatment of peripheral CTOs in the US.11
What Does It Cost?
The cost of the Soundbite Crossing system is currently unavailable.
What is the Evidence?
As the Soundbite system is still in development for coronary applications, there is no specific evidence regarding its clinical effectiveness in coronary CTOs. However, clinical outcomes from completed studies in peripheral CTOs may provide a basis for predicting its success.
In a prospective, single-arm feasibility study, Soundbite Medical Solutions investigated the safety and efficacy of the Soundbite system in patients with infrainguinal CTOs.1 The study used a 0.018 inch wire to deliver shock waves to peripheral CTOs, which is slightly larger than the wire being developed for coronary applications.1 The primary end point of device success, defined as, among various other outcomes, complete crossing of the target CTO lesion and freedom from major adverse events for 30 days post-procedure, was achieved in 91.9% of patients.1 The authors noted several limitations of the study, such as the small cohort of 37 patients and the lack of intravascular imaging during the procedure.1
Safety
In the Soundbite feasibility study for peripheral infrainguinal CTOs, 13.5% of patients presented angiographic evidence of arterial perforation.1 However, none of them required treatment.1
Related Developments
The Crosser catheter by Bard Peripheral Vascular has also emerged as a treatment option for peripheral CTOs. This option uses a higher frequency generator but similarly sized wire as the Soundbite system.12 The Crosser catheter is commercially available in the US.13
Shockwave Medical’s intravascular lithotripsy device uses low-pressure balloon dilatation with shock waves to penetrate and cross calcified plaques in both peripheral and coronary vasculature.9 By using lower pressures rather than traditional balloon-based procedures, intravascular lithotripsy is thought to minimize vascular injury.5 Currently, coronary intravascular lithotripsy catheters are commercially available in Europe but are limited to investigational use in the US.14 Shockwave Medical’s largest study to date (DISRUPT CAD III) includes 392 patients and is further examining the coronary applications of this technology. DISRUPT CAD III is expected to be completed by 2022.15
Looking Ahead
Overall, Soundbite Medical Solutions’ Soundbite Crossing system may be a promising non-surgical treatment option for patients with CTOs, but its evidence for use in coronary heart disease is lacking. As of March 2020, Soundbite Medical Solutions has received FDA approval to commence the ACTIVE study on coronary applications of the Soundbite Crossing system in the US.16 The study is expected to be conducted in 154 patients, with primary end points being device success and freedom from related major adverse events 48 hours post-procedure.17 Considering the short follow-up period, the long-term clinical effectiveness of the Soundbite Crossing system will not be an outcome of this study. As all Soundbite system investigations have been non-comparative, studies directly comparing the clinical effectiveness of shock wave technology to conventional angioplasty methods would further clarify its place in therapy.
Author: Diksha Kumar
Reference
- Brodmann M, Therasse E, Benko A, Riel LP, Dion S, Genereux P, et al. Recanalization of CTOs with SoundBiteTM Active Wire. J Cardiovasc Surg. 2018;59(4):529-537.
- Khatri J, Abdallah M, Ellis S. Management of coronary chronic total occlusion. Cleve Clin J Med. 2017;84(12 suppl 3):27-38.
- Heart and Vascular Institute, University of Pittsburgh Medical Center. Chronic coronary total occlusion - Treatment. Pittsburgh (PA): University of Pittsburgh Schools of Health Sciences: https://www.upmc.com/services/heart-vascular/conditions-treatments/chronic-coronary-total-occlusion#treatment. Accessed 2020 Feb 14.
- Heart Center. Chronic Total Occlusion Percutaneous Coronary Intervention. Boston (MA): Massachusetts General Hospital; 2020: https://www.massgeneral.org/heart-center/treatments-and-services/chronic-total-occlusion-percutaneous-coronary-intervention-cto-pci. Accessed 2020 Feb 21.
- Brinton TJ, Ali ZA, Hill JM, Meredith IT, Maehara A, Illindala U, et al. Feasibility of Shockwave Coronary Intravascular Lithotripsy for the Treatment of Calcified Coronary Stenoses. Circulation. 2019;139(6):834-836.
- National Heart, Lung, and Blood Institute. Coronary artery bypass grafting. Bethesda (MD): U.S. Department of Health and Human Services; 2020: https://www.nhlbi.nih.gov/health-topics/coronary-artery-bypass-grafting. Accessed 2020 Mar 3.
- Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, et al. Current Perspectives on Coronary Chronic Total Occlusions: The Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59(11):991-997.
- Heart disease in Canada. Ottawa (ON): Government of Canada; 2017: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/heart-disease-canada.html. Accessed 2020 Mar 17.
- Aksoy A, Salazar C, Zimmer S, Escaned J, Nickenig G, Sinning JM, et al. TCT-653 Intravascular Lithotripsy for Lesion Preparation in Calcified Coronary Lesions: First Data of Prospective, Observational Multicenter Registry. J Am Coll Cardiol. 2019;74 (13 Supplement):B641.
- SoundBite announces Health Canada approval of the SoundBite™ Crossing System – Peripheral. Montreal (QC): SoundBite Medical Solutions; 2020: https://soundbitemedical.com/soundbite-announces-health-canada-approval-of-the-soundbite-crossing-system-peripheral/. Accessed 2020 Feb 14.
- SoundBite receives FDA 510(k) Clearance of the SoundBite™ Crossing System – Peripheral. Montreal (QC): SoundBite Medical Solutions; 2020: https://soundbitemedical.com/soundbite-receives-fda-510k-clearance-of-the-soundbite-crossing-system-peripheral/. Accessed 2020 Feb 14.
- Laird J, Joye J, Sachdev N, Huang P, Caputo R, Mohiuddin I, et al. Recanalization of infrainguinal chronic total occlusions with the crosser system: results of the PATRIOT trial. J Invasive Cardiol. 2014;26(10):497-504.
- Crosser™ CTO recanalization catheters. Tempe (AZ): Bard Peripheral Vascular, Inc.; 2018: https://www.crbard.com/Peripheral-Vascular/en-US/Products/Crosser-CTO-recanalization-catheters. Accessed 2020 Mar 13.
- Shockwave receives FDA Breakthrough Device designation for the Coronary IVL System. Santa Clara (CA): Shockwave Medical, Inc. ; 2019: https://shockwavemedical.com/about/press-releases/shockwave-receives-fda-breakthrough-device-designation-for-the-coronary-ivl-system/. Accessed 2020 Mar 20.
- Shockwave Medical Inc. NCT03595176: Disrupt CAD III with the Shockwave Coronary IVL System. Clinicaltrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019: https://clinicaltrials.gov/ct2/show/NCT03595176. Accessed 2020 Feb 14.
- SoundBite receives FDA IDE Approval for the ACTIVE Clinical Study with the SoundBite™ Crossing System – Coronary. Montreal (QC): Soundbite Medical Solutions, Inc. ; 2020: https://soundbitemedical.com/soundbite-receives-fda-ide-approval-for-the-active-clinical-study-with-the-soundbite-crossing-system-coronary/. Accessed 2020 Mar 13.
- Soundbite Medical Solutions. NCT03521804: Safety and efficacy study of the SoundBite™ Crossing System With ACTIVE Wire in Coronary CTOs. (ACTIVE). Clinicaltrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2020: https://clinicaltrials.gov/ct2/show/NCT03521804. Accessed 2020 Mar 13.
