Dernière mise à jour : 21 mars 2017
Détails
État du projet:
Terminé
Gamme de produits:
Analyse prospective
Numéro de projet :
EH0044-000
(À l'exception du résumé, le texte n'est disponible qu'en en anglais)
Résumé

- Le Probuphine est le premier implant sous-cutané utilisé dans traitement d’entretien pour contrer la dépendance aux opiacés. Il est conçu pour fournir de façon continue et constante des niveaux de buprénorphine dans le sang pendant jusqu’à six mois après une simple intervention ambulatoire réalisée dans un bureau médical. Selon le fabricant, le Probuphine pourrait régler les problèmes associés à l’autoadministration de la buprénorphine sublinguale, comme l’observance précaire, l’adhésion, l’abus, le détournement et l’exposition accidentelle aux enfants.
- En mai 2016, la US Food and Drug Administration (FDA) a approuvé les implants de Probuphine comme traitement de gestion de la dépendance aux opiacés chez les patients qui avaient atteint et maintenu une stabilité clinique prolongée à l’aide de doses faibles à modérées d’un produit transmuqueux à base de buprénorphine (c’est-à-dire des doses de moins de 8 mg par jour de Subutex ou de Suboxone en comprimé sublingual ou un équivalent générique). L’usage du Probuphine n’est pas recommandé après deux cycles de traitement de six mois. Une demande de permission de commercialiser devrait être déposée à Santé Canada dans les 12 mois (à compter de décembre 2016).
- Les résultats de deux essais de phase III contrôlés par placébo à double insu ont révélé que le Probuphine serait plus efficace que l’implant de placébo dans la réduction de l’usage d’opiacés illicites pendant un traitement de six mois chez les nouveaux participants au traitement. Une quantité significativement plus importante de patients du groupe utilisant le Probuphine a terminé le traitement (65,7 % dans le premier essai et 64,0 % dans le second essai, contre 30,9 % dans le premier essai et 25,9 % dans le second essai avec placébo; p < 0,001 pour les deux essais). Un implant supplémentaire a été fourni aux patients qui dépassaient certains critères donnés pour une utilisation plus importante de buprénorphine-naloxone afin de gérer les symptômes de sevrage et de manque. Dans le groupe avec Probuphine, un implant supplémentaire a été reçu par 20,3 % des patients dans le premier essai et par 21,9 % des patients du deuxième essai. Dans le groupe placébo, 58,2 % des patients du premier essai et 38,8 % des patients du second essai ont eu besoin d’un implant supplémentaire.
- Un essai de phase III à double insu contrôlé en double feinte par une substance active a révélé que le Probuphine n’était pas inférieur à la buprénorphine sublinguale en ce qui concerne les principaux résultats d’au moins quatre des six mois sans usage d’opiacés illicites chez les patients présentant une dépendance aux opiacés qui avaient été stabilisés précédemment à l’aide d’une dose faible à modérée (≤ 8 mg/jour) de buprénorphine sublinguale. La majorité des participants de chacun des groupes (93,1 % de ceux qui recevaient du Probuphine et 94,4 % qui recevaient de la buprénorphine sublinguale) a terminé l’essai. Cependant, quelques patients des deux groupes ont eu besoin de buprénorphine sublinguale supplémentaire (17,9 % de ceux recevant du Probuphine contre 14,6 % de ceux recevant de la buprénorphine sublinguale).
- On ne sait pas clairement comment le besoin de traitement supplémentaire de buprénorphine sublinguale démontré dans les essais de phase III peut influencer la capacité du Probuphine de réduire les abus et l’exposition accidentelle aux enfants, surtout si l’usage en est limité à un sous-groupe de patients dont la dépendance aux opiacés est cliniquement stable.
- Trois essais de phase III et deux études de prolongation ouvertes qui évaluaient le Probuphine pendant des cycles allant jusqu’à deux traitements de six mois ont révélé que dans l’ensemble les événements indésirables au site de l’implant étaient comparables entre les groupes traités. La plupart des événements indésirables rapportés en lien au site de l’implant étaient des douleurs, du prurit et de l’érythème se manifestant de façon mineure et se réglant sans nécessiter de traitement. Il n’y avait aucune preuve de retrait ou de tentative non prévue de retrait de l’implant ni de cas de migration de l’implant.
- Tous les prestataires de soins de santé aux É.-U. doivent suivre une formation pratique sur les procédures d’insertion et de retrait; ils doivent également être certifiés au programme restreint nommé Probuphine Risk Evaluation and Mitigation Strategy program (REMS) avant de prescrire du Probuphine ou de réaliser des insertions et des retraits.
Background
Opioid use disorder (also known as opioid dependence), defined as a problematic pattern of opioid use leading to clinically significant impairment or distress, is a growing public health concern in Canada that is associated with significant morbidity and mortality.1-3 Individuals with opioid use disorder are at greater risk for incarceration, blood-borne infections, and fatal overdose.4 In addition, babies born to mothers who used opioids during pregnancy are at increased risk for neonatal opioid withdrawal syndrome, a life-threatening condition if not promptly recognized.5 While heroin has historically been the most commonly misused opioid, nonmedical use of prescription opioid analgesics (such as morphine, hydromorphone, and fentanyl) is now the dominant form of opioid misuse.6 “Misuse” has been defined as “the intentional or unintentional use of a prescribed medication in a manner that is contrary to directions, regardless of whether a harmful outcome occurs.”7
Canada is now the world’s second largest per capita consumer of prescription opioids.8 A total of 4.3 million Canadians older than 15 years of age (representing 14.9% of the total population) reported using opioid pain relievers in the past year, according to a Government of Canada survey conducted in 2013.9 Among users of opioid pain relievers, 2.3% (99,000 Canadians, representing 0.3% of the total population) reported misusing them. A joint report by the Canadian Institute for Health Information (CIHI) and the Canadian Centre on Substance Abuse found that between 2007-2008 and 2014-2015, the rate of hospitalizations due to opioid poisoning in Canada increased by more than 30%.10 Currently, opioid poisoning results in more than 13 hospitalizations a day in Canada, with an average length of stay of eight days. The report also found that the rate of emergency department visits increased by 53% in Alberta and by 22% in Ontario between 2010-2011 and 2014-2015. The number of people enrolled in methadone maintenance treatment programs in Ontario increased from approximately 7,800 in 2001 to more than 35,000 in 2011.11 Research from the Centre for Addiction and Mental Health indicates that fatalities from opioid overdose in Ontario increased by 24% from 2010 to 2013.12 Recently, there have also been a growing number of deaths specifically due to fentanyl.13 The Canadian Centre on Substance Abuse reported that between 2009 and 2014, there were 655 fentanyl-related deaths in Canada.13 The significant burden of prescription drug misuse on the Canadian health care system has triggered multipronged efforts in different jurisdictions to identify key factors contributing to the epidemic as well as to address the unmet treatment needs of patients with opioid use disorder.14
There are two medications approved for the maintenance treatment of opioid use disorder in Canada: methadone and buprenorphine-naloxone (either the brand name version Suboxone or its generic equivalents). When taken as prescribed, these medications alleviate withdrawal symptoms and diminish cravings without the typical euphoric effects of opioids.2 When buprenorphine-naloxone is administered sublingually, the naloxone component is not significantly absorbed and the opioid effect of the buprenorphine is not completely blocked. However, when buprenorphine-naloxone is administered parenterally or intranasally, the naloxone is significantly absorbed and blocks the opioid effect of buprenorphine, which is intended to discourage misuse of the drug as administered through these routes. Buprenorphine on its own (Subutex) is available only through the Health Canada Special Access Program for specific clinical situations where naloxone is contraindicated, such as pregnancy.1 Buprenorphine-naloxone has been shown to be as effective as methadone in reducing illicit opioid use, although less effective for retention in treatment, with a potentially improved safety profile and a reduced risk for overdose or diversion, relative to methadone.1,2,15-17 Buprenorphine-naloxone also offers the possibility to improve access to treatment for opioid use disorder in rural communities and remote regions due to factors such as its potentially improved safety profile; fewer prescribing and dispensing restrictions compared with methadone (although not in all jurisdictions); and the potential for flexible at-home dosing, or dosing every second day.1,2,18,19
However, despite the potential advantages of buprenorphine-naloxone (Suboxone or its generic equivalents) compared with methadone, currently available formulations require supervised administration early in treatment or self-administration once clinically stable on a daily basis.20 Thus, particularly in an office-based treatment setting where medication administration is unsupervised, effectiveness relies on long-term patient adherence to daily treatment and there is a risk of diversion and misuse.20 Furthermore, there is also a risk of accidental pediatric exposure. The US Centers for Disease Control and Prevention reported that between 2010 and 2011, 9.5% of emergency hospitalizations for drug ingestion among children younger than six years of age were caused by buprenorphine-naloxone.21 These limitations have spurred interest in the development of alternative formulations.
The Technology
 Probuphine is the first subdermal implant designed to provide continuous, non-fluctuating, blood levels of buprenorphine for up to six months following a single procedure.22 Probuphine is marketed in the US by Braeburn Pharmaceuticals under a licensing agreement with Titan Pharmaceuticals Inc.23 Probuphine utilizes a proprietary continuous drug delivery system, consisting of a match-stick sized solid rod made from a mixture of ethylene-vinyl acetate and the drug substance.24 The resulting product is a solid matrix that is placed subcutaneously, normally in the inner part of the upper arm in an office procedure, and is removed at the end of the treatment period. There is no reservoir, which reduces the risk of “drug dumping,” which is when a reservoir membrane ruptures and suddenly releases a large amount of a drug into the bloodstream.
Probuphine is the first subdermal implant designed to provide continuous, non-fluctuating, blood levels of buprenorphine for up to six months following a single procedure.22 Probuphine is marketed in the US by Braeburn Pharmaceuticals under a licensing agreement with Titan Pharmaceuticals Inc.23 Probuphine utilizes a proprietary continuous drug delivery system, consisting of a match-stick sized solid rod made from a mixture of ethylene-vinyl acetate and the drug substance.24 The resulting product is a solid matrix that is placed subcutaneously, normally in the inner part of the upper arm in an office procedure, and is removed at the end of the treatment period. There is no reservoir, which reduces the risk of “drug dumping,” which is when a reservoir membrane ruptures and suddenly releases a large amount of a drug into the bloodstream.
Following the injection of a local anesthetic, four implants are placed subdermally in the inside of the upper arm in an outpatient, office-based procedure, and removed in a similar manner at the end of the six-month treatment period.25 The drug is released continuously, resulting in a stable, non-fluctuating level of the drug in the bloodstream, without the peak-and-trough levels that occur with oral dosing. Each implant contains 74.2 mg of buprenorphine (equivalent to 80 mg of buprenorphine hydrochloride), for a total of 320 mg of buprenorphine being implanted at once.25 Four Probuphine implants yield plasma buprenorphine concentrations at a range (0.5 ng/mL to 1 ng/mL) comparable with the average plasma concentrations observed following daily doses with 4 mg/day to 8 mg/day of Suboxone tablets or equivalent transmucosal buprenorphine products.25
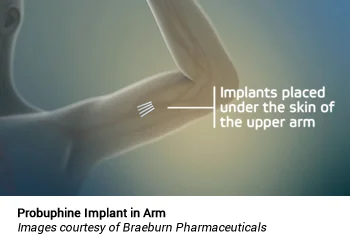 Probuphine subdermal implants are intended to be in place for six months of treatment. If continued treatment is desired, new implants may be inserted in the opposite arm.25 If new implants are not inserted on the same day as the removal of implants, patients should be maintained on their previous dosage of transmucosal buprenorphine (i.e., the dose from which they were transferred to Probuphine treatment) before switching back to Probuphine treatment. Following the insertion of implants in each arm for a maximum of two six-month treatment cycles, patients should be transitioned back to a transmucosal buprenorphine-containing product for continued treatment.25 Since there is no clinical experience with the insertion of implants beyond a single insertion in each arm, the US prescribing information states that additional cycles of treatment should be considered only if the potential benefits of continuing treatment outweigh the potential risks of additional insertion and removal procedures.25
Probuphine subdermal implants are intended to be in place for six months of treatment. If continued treatment is desired, new implants may be inserted in the opposite arm.25 If new implants are not inserted on the same day as the removal of implants, patients should be maintained on their previous dosage of transmucosal buprenorphine (i.e., the dose from which they were transferred to Probuphine treatment) before switching back to Probuphine treatment. Following the insertion of implants in each arm for a maximum of two six-month treatment cycles, patients should be transitioned back to a transmucosal buprenorphine-containing product for continued treatment.25 Since there is no clinical experience with the insertion of implants beyond a single insertion in each arm, the US prescribing information states that additional cycles of treatment should be considered only if the potential benefits of continuing treatment outweigh the potential risks of additional insertion and removal procedures.25
Due to the risk of serious complications resulting from improper insertion or removal of the implants, all health care providers in the US must successfully complete a live training program on the insertion and removal procedures and become certified in the restricted program called the Probuphine Risk Evaluation and Mitigation Strategy (REMS) program, before performing insertions or prescribing Probuphine implants.25
According to the manufacturers, Probuphine offers the potential to address issues associated with oral buprenorphine such as poor compliance, adherence, misuse, diversion, and accidental pediatric exposure.23 Additionally, this approach may provide better access to treatment for individuals in remote communities who have minimal access to clinical facilities or methadone programs.26 However, further research is required to confirm these proposed benefits.
Regulatory Status
Probuphine has not been approved for sale in Canada. According to Knight Therapeutics, the submission to Health Canada is planned within the next 12 months (as of September 2016) (Knight Therapeutics Inc., Westmount, QC: personal communication, 2016 Sep). Knight Therapeutics has the exclusive rights to market Probuphine in Canada under a sublicense agreement with Braeburn Pharmaceuticals.22
In May 2016, the US Food and Drug Administration (FDA) approved Probuphine implants for the maintenance treatment of opioid dependence in patients who have achieved and sustained prolonged clinical stability on low-to-moderate doses of a transmucosal buprenorphine-containing product (i.e., doses of no more than 8 mg per day of Subutex or Suboxone sublingual tablet or generic equivalent).25,27 The period of time a patient should be clinically stable is not specified in the indication. Probuphine was not approved for new entrants to treatment or for patients who have not achieved and sustained prolonged clinical stability while being maintained on buprenorphine 8 mg per day or less of a Subutex or Suboxone sublingual tablet or generic equivalent.25
Patient Group
Although the true prevalence of opioid use disorder in Canada is unknown, there are an estimated 75,000 to 125,000 injection drug users (the vast majority of whom inject opioids such as heroin) and 200,000 people with prescription opioid use disorder.28,29 The misuse of opioids leads to tolerance to the euphoric effects of the opioid, cravings, and a physiological withdrawal state when use is tapered quickly or stopped.30 Opioid use disorder is characterized by the compulsive, prolonged administration of opioid substances despite the treatment of acute withdrawal.3 As a result, individuals experience physical dependence, medical and psychological problems, and social dysfunction.6 Opioid use disorder increases the risk for mortality, local and systemic infections (including cellulitis, endocarditis, osteomyelitis, tuberculosis, and pneumonia), infection with a blood-borne pathogen (such as HIV, hepatitis B, and hepatitis C), and narcotic bowel syndrome (an increase in abdominal pain associated with continued or escalating dosages of opioids).4 Opioid users also have an increased rate of accident-related injuries compared with the general population.4
According to the US prescribing information, the Probuphine approved-patient population are those individuals who have achieved and sustained prolonged clinical stability on low-tomoderate doses of a transmucosal buprenorphine-containing product (i.e., doses of no more than 8 mg per day). There are currently no estimates for the number of patients who are clinically stable on maintenance therapy for opioid use disorder in Canada. Probuphine could improve quality of life by reducing “pill burden” and “pharmacy visits.”31 According to the clinical expert consulted for this bulletin, Probuphine could potentially be used for clinically stable patients who are planning on discontinuing therapy within six to 12 months. (Dr. Peter Selby, Director, Medical Education; Clinician Scientist, Addictions, Centre for Addiction and Mental Health, Toronto, ON: personal communication, 2017 Feb).
Current Practice
Opioid use disorder is a chronic, relapsing illness that requires long-term maintenance treatment.2 Guidelines strongly recommend against a strategy involving withdrawal management alone (i.e., detoxification without transition to longer-term treatment), since this approach has been associated with elevated rates of relapse, transmission of infectious diseases, criminal activity, and an increased risk of mortality due to overdose following the loss of tolerance to the effects of opioids following complete discontinuation.1,2,4 Maintenance treatment of opioid use disorder involves a comprehensive approach that combines approved opioid agonist mediations with counselling, psychosocial rehabilitation, and other behavioural therapies to reduce opioid drug misuse by decreasing cravings, addressing withdrawal symptoms, and promoting functional recovery in everyday living.2 Treatment goals focus more on functional recovery than on abstinence only outcomes. Maintenance treatment for two years is recommended.32-35 However, according to the clinical expert consulted for this bulletin, at least six months of complete abstinence should be achieved before any tapering is attempted. (Dr. Peter Selby, Director, Medical Education; Clinician Scientist, Addictions, Centre for Addiction and Mental Health, Toronto, ON: personal communication, 2017 feb). For individuals with successful and sustained response to opioid agonist treatment, who wish to stop taking the medication, a slow tapering of therapy over 12 months could be considered.2
Treatment with methadone has been considered the gold standard for maintenance treatment of opioid use disorder in Canada for decades.36 However, because of the narrow therapeutic index in some patients, especially during the first few weeks of treatment, methadone can be prescribed only by physicians who hold a methadone exemption granted by Health Canada.1,37 Depending on the jurisdiction, for the first two to six months of therapy, methadone is dispensed under daily witnessed ingestion at specialized drug treatment clinics or pharmacies. This is has proved a barrier to many patients, especially those in rural communities.1,18
Until recently, buprenorphine-naloxone was generally covered on provincial drug plans only if treatment with methadone failed, was contraindicated, or was not medically tolerated.14 In October 2016, the Ontario Ministry of Health announced that buprenorphine-naloxone would be added to its drug formulary as a regular benefit, making it available as a first-line treatment option for opioid use disorder.38 British Columbia added buprenorphine-naloxone to its drug formulary as a regular benefit in October 2015.36 Some provinces require physicians to hold a methadone exemption and complete additional training before prescribing buprenorphine-naloxone.2 However, other provinces, including Alberta, British Columbia, and Ontario, no longer require physicians to hold a methadone exemption before being able to prescribe buprenorphine-naloxone.39-41
Current guidelines recommend maintenance therapy with buprenorphine-naloxone as first-line treatment rather than methadone because of its many potential advantages, which include a potentially improved safety profile, a lower risk of overdose or diversion, and the potential for more flexible at-home dosing.2 Generally, the average maintenance dose of buprenorphine is between 8 mg/day and 12 mg/day.42,43 While there are no established protocols for take-home dosing of buprenorphine-naloxone, Health Canada recommends that the administration of all doses be observed for at least the first two months of treatment, with the exception of holidays and weekends.2 Methadone may be considered first-line when contraindications to buprenorphine-naloxone exist or when a challenging induction is anticipated because of prior treatment failures with buprenorphine-naloxone, a history of severe withdrawal symptoms, or an expected need for high maintenance treatment doses.2 At least eight weeks of program enrolment, with at least the preceding week of no substance use before the first take-home dose, is required before take-home doses of methadone are considered.35 Alternative approaches for patients with opioid use disorder who respond poorly to other maintenance treatments include slow-release oral morphine and injectable diacetylmorphine (also known as heroin).2 In September 2016, Health Canada amended regulations allowing doctors to prescribe heroin under the Special Access Programme (SAP) to individuals with severe opioid use disorder for whom other treatment approaches have repeatedly failed.44
Methods
A peer-reviewed literature search was conducted using the following bibliographic databases: MEDLINE, PubMed, Embase, and the Cochrane Library. Grey literature was identified by searching relevant sections of the Grey Matters checklist (https://www.cadth.ca/grey-matters). No methodological filters were applied. The search was limited to English-language documents with no date limit applied. Regular alerts were established to update the search until October 31, 2016.
Phase III randomized controlled trials, published in full or presented as unpublished data from industry communications and conference abstracts, reporting the clinical efficacy and safety of buprenorphine implants for the treatment of opioid use disorder were selected for inclusion in “The Evidence” section of this bulletin. Unpublished open-label extension trials of phase III trials evaluating the safety of buprenorphine implants were also included the “Adverse Event” section. Meta-analyses, case reports, editorials, letters, and narrative literature reviews were excluded.
The Evidence
Three phase III trials have evaluated the efficacy of Probuphine for maintenance treatment in patients with opioid use disorder.42,43,45 Details of these trials are summarized in Table 1. The primary outcome in all three phase III trials was the reduction in illicit opioid use based on urine testing. However, a poor correlation has been found between urine drug screen results and functional outcomes such as changes in quality of life in previous studies.46 All three trials reported completion of treatment, a clinically meaningful measure that has been correlated with quality of life, as a secondary outcome.47 In addition to the three published phase III trials, an ongoing, openlabel, crossover study is evaluating the effect of Probuphine on sleep disturbances among patients with clinically stable opioid use disorder.48
Table 1: Summary of Probuphine Implant Phase III Efficacy Trials
| Study | Main Inclusion/Exclusion Criteria | Study Details | Baseline Characteristics |
|---|---|---|---|
| PRO-80542 N = 163 |
Inclusion:
Exclusion:
|
Design: Multi-centre, randomized, placebo-controlled, double-blind with open-label active comparator arm Intervention: Four 80 mg buprenorphine implants Comparator: Four placebo implants Open-label comparator: Sublingual buprenorphinenaloxone Setting: 20 US addiction treatment centres Duration: 24 weeks |
Mean age, years: 35.8 to 39.3 Caucasian, N (%): 122 (74.8) Male, N (%): 112 (68.7%) Primary opioid of misuse, N (%): Heroin 103 (63.2) No previous pharmacotherapy for opioid dependence, N (%): 124 (76.1) |
| Pro-81445 N =177 |
Inclusion:
Exclusion:
|
Design: Multi-centre, randomized, activecontrolled, double-blind, doubledummy Intervention: Four 80 mg Probuphine implants + sublingual placebo Comparator: Sublingual buprenorphine + four placebo implants Setting: 21 US office-based outpatient treatment sites Duration: 24 weeks |
Mean age, years (SD): 39.0 (11.0) Caucasian, N (%): 167 (94.9) Male, N(%): 104 (59.1) Primary opioid of misuse, N (%): Prescription opioids: 131 (74.4) Highest education level achieved, N (%): GED or high school diploma: 95 (54.0) Four-year college degree: 38 (21.6) Current employment status, N (%): Full time (≥ 35 hours/week): 97 (55.1) Unemployed: 32 (18.2) Buprenorphine dose at randomization, N (%): 8 mg/day 128 (72.7) ≤ 4 mg/day 36 (20.4%) Mean time of treatment with buprenorphine, years (SD): 3.5 (2.58) |
| PRO-80643 N = 287 |
Inclusion:
Exclusion:
|
Design: Multi-centre, randomized, placebo-controlled, double-blind with open-label active comparator arm Intervention: Four 80 mg buprenorphine implants Comparator: Four placebo implants Open-label comparator: Sublingual buprenorphine-naloxone Setting: 20 US addiction treatment centres Duration: 24 weeks |
Mean age, years: 35.2 to 36.4 Caucasian, N (%): 237 (82.6) Male, N(%): 175 (60.9) Primary opioid of misuse, N (%): Heroin 179 (62.4) No previous pharmacotherapy for opioid dependence, N (%): 125 (43.6) |
DSM-IV = Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).
Placebo-Controlled Trials:
PRO-805 was the first randomized, placebo-controlled trial designed to evaluate Probuphine implants in new entrants to maintenance treatment of opioid use disorder.42 Before randomization, eligible patients entered into an open-label induction phase with a fixed dose of 12 mg/day to 16 mg/ day of sublingual buprenorphine-naloxone tablets for at least three consecutive days, to ensure the drug could be safely administered. A total of 163 patients were randomized in a 2:1 ratio to receive four Probuphine implants (n = 108) or four placebo implants (n = 55). Supplemental buprenorphine-naloxone use was permitted in both groups if significant withdrawal symptoms or cravings were encountered. An additional implant was provided for participants if they required supplemental buprenorphine-naloxone for three or more days per week in two consecutive weeks or eight or more days over four consecutive weeks. All participants received individual drug counselling.
The primary outcome was the percentage of urine samples that were negative for illicit opioids for weeks 1 through 16. The secondary outcome was the percentage of urine samples that were negative for illicit opioids for weeks 17 through 24. Other outcomes included the proportion of treatment failures (i.e., the need for a fifth implant and subsequently requiring supplemental buprenorphine-naloxone either ≥ 3 days per week for two consecutive weeks or ≥ 8 days over four weeks) and the proportion of study completers. All missing urine samples were assumed to be positive, as were urine samples for patients who were withdrawn from the study due to nonadherence with the study protocol.
Significantly more patients in the Probuphine group completed treatment (65.7% versus 30.9% with placebo; P < 0.001). Treatment with Probuphine significantly increased the mean proportion of urine samples per participant testing negative for illicit opioids during weeks 1 to 16 compared with placebo (40.4%; 95% confidence interval [CI], 34.2% to 46.7% with Probuphine versus 28.3%; 95% CI, 20.3% to 36.3% with placebo; P = 0.04). For the period between 17 and 24 weeks, no specific data were provided other than that the difference remained significant (P < 0.001). Additional implants were received in 22 (20.3%) patients in the Probuphine group and 32 (58.2%) patients in the placebo group during the 24-week study period. No patients in the Probuphine group met the definition of having had treatment that failed, while 17 (30.9%) placebo patients were classified as having had treatment that failed.
Several limitations of PRO-805 are important to consider. As expected, attrition was high in the placebo group, with 30.9% completing treatment. With the assumption that missing urine samples were considered positive and considering that the greater proportion of missing samples were in the placebo group, the treatment difference observed between groups for the primary outcome could have been artificially inflated.49 Second, the clinical significance of the primary outcome measure in this study (the proportion of illicit opioid–negative urine samples in the first 16 weeks) has been questioned, particularly in the evaluation of a long-term treatment.49 Third, 22 (20.4%) patients in the Probuphine group required a fifth implant, and the mean steady state plasma concentrations over weeks 4 through 24 from the four or five implants in the Probuphine group were relatively low. Furthermore, the mean percentage of urine samples that were still positive for illicit opioids over the full 24-week study period was 63.4% in the Probuphine group versus 77.6% for the placebo group.
PRO-806 was the second randomized, double-blind, placebo-controlled trial designed to evaluate the efficacy of Probuphine in new entrants to treatment of opioid use disorder.43 The primary objective was to confirm efficacy of Probuphine implants relative to placebo over 24 weeks of treatment. A secondary objective was to establish the non-inferiority of Probuphine implants to buprenorphine-naloxone. After an open-label induction phase with buprenorphine-naloxone at a target dose of 12 mg/day to 16 mg/day, a total of 287 participants were randomized in a 2:1:2 ratio to receive four Probuphine implants (n = 114) or four placebo implants (n = 54) or openlabel buprenorphine-naloxone (12 mg/day to 16 mg/day once daily) (n = 119). During the 24-week study, all patients could receive supplemental sublingual buprenorphine-naloxone for withdrawal symptoms and cravings. Patients in the Probuphine and placebo implant groups could also receive an additional implant upon satisfying the same criteria set in PRO-805. If patients met the criteria for a dose increase after receiving an additional implant, treatment was considered to have failed and those patients were withdrawn from the study. Drug counselling sessions were also provided. Urine samples were collected three times a week. All missing urine samples were assumed to be positive, as were urine samples for patients who were withdrawn from the study because of nonadherence with the study protocol.
The primary efficacy outcome was the percentage of urine samples collected from weeks 1 to 24 that were negative for opioids, expressed as cumulative distribution function curve. Secondary efficacy outcomes were the percentage of urine samples that were negative for opioids during weeks 1 to 16 and 17 to 24. Additional secondary measures included the proportion of study completers. For the non-blinded, open-label comparison between Probuphine and buprenorphine-naloxone, non-inferiority was established if the lower bound of the 95% CI for the mean difference between proportions of urine samples negative for opioids between the two groups was greater than –15%. The non-inferiority margin was based on input from clinical experts and previous studies.
Patients in the Probuphine group had a higher completion rate relative to placebo (64.0% versus 25.9%; P < 0.0001). Additional implants were received by 25 (21.9%) patients in the Probuphine group and 21 (38.8%) patients in the placebo implant group. Supplemental sublingual buprenorphine-naloxone was used by 45 (39.5%) patients in the Probuphine group, 36 (66.7%) patients in the placebo implant group, and seven (5.9%) patients in the sublingual buprenorphine-naloxone group.
The primary outcome, comparing Probuphine with the placebo implant group on the cumulative distribution function curve of the percentage of opioid–negative urine samples from weeks 1 to 24, showed a significant difference between the groups (P < 0.0001). Mean proportions of urine samples that were negative for opioids were higher in the Probuphine group than the placebo implant group (31.2%; 95% CI, 25.3 to 37.1 versus 13.4%; 95% CI, 8.3 to 18.6, respectively). Patients receiving Probuphine were more likely to have at least 50% of urine samples negative for opioids (27% versus 6% for the placebo implant). Statistically significant differences were also observed for all secondary outcomes. Probuphine was noninferior to sublingual buprenorphine-naloxone based on percentage of urine samples negative for opioids (mean proportions of urine samples negative for opioids for Probuphine 31.2%; 95% CI, 25.3 to 37.1 versus 33.5% 95% CI, 27.3 to 39.6 for sublingual buprenorphine-naloxone for the difference of proportions of –10.7 to 6.2).
Limitations of the PRO-806 study are similar to those in PRO-805. Additional implants were received by 25 (21.9%) patients in the Probuphine group. Furthermore, the mean percentage of urine samples that were still positive for illicit opioids was 68.8% in the Probuphine group versus 86.6% for the placebo group during the full 24-week study period.
The original Probuphine submission in 2013 for FDA approval presented the results of PRO-805 and PRO-806. The requirement for supplemental sublingual buprenorphine-naloxone and additional implants in the Probuphine groups in both trials indicated that the dose provided was too low to provide effective treatment for patients new to buprenorphine treatment.50 The results were determined by the FDA to not be sufficiently robust to support approval, and exploration of higher doses or evaluation of a different patient population with lower buprenorphine dose requirements were recommended.50 Hence, a study focusing on a subpopulation of patients who were stable on lower doses of buprenorphine was designed for further evaluation of efficacy.
Active-Controlled Trial
Approval of Probuphine in the US was based on the results of PRO-814, a randomized, active-controlled, double-blind, double-dummy study designed to determine whether six-month Probuphine implants were noninferior to daily sublingual buprenorphine as maintenance treatment in patients with opioid use disorder who were clinically stable on a lower dose (≤ 8 mg/day) of sublingual buprenorphine.45 A total of 177 participants were randomized in a 1:1 ratio to continue treatment with a sublingual buprenorphine product with or without naloxone at the same dose (≤ 8 mg/day) with four placebo implants (n = 90) or four Probuphine implants with sublingual placebo (n = 87). Four implants were expected to yield plasma buprenorphine concentrations at a range comparable to 8 mg/day or less of sublingual buprenorphine, although this was not measured in the study. As-needed unblinded supplemental sublingual buprenorphine was permitted as clinically indicated during the study; a fifth implant was not permitted. Urine samples were obtained for drug testing at scheduled monthly visits and four times randomly for a total of 10 urine samples.
Efficacy outcome analyses were based on the pre-defined intention-to-treat population consisting of all randomized patients who received study medication and provided efficacy data. The primary efficacy outcome was the difference in the proportion of responders, defined as patients with at least four of six months with no evidence of illicit opioid use (based on a negative urine test and no self-report of illicit opioid use) by treatment group. The study tested non-inferiority established for the lower bound of the 95% CI greater than –0.20 within the intention-to-treat population (one-sided testing with a significance threshold of P < 0.025). The non-inferiority margin was based on input from clinical experts and a literature review.45,51 A non-pre-specified test was then performed for superiority. Secondary outcomes included cumulative percentage of negative opioid urine results at six months, treatment retention, and time to first illicit opioid use. Opioid craving, withdrawal, and supplemental use of sublingual buprenorphine were also measured.
A total of 81 (93.1%) patients who received Probuphine and 84 (94.4%) patients who received sublingual buprenorphine completed the study. Fifteen (17.9%) patients in the Probuphine group and 13 (14.6%) patients in the sublingual buprenorphine group received supplemental sublingual buprenorphine (P > 0.05). A total of 81 (96.4%) patients in the Probuphine group responded to therapy versus 78 (87.6%) patients in the sublingual buprenorphine group. The treatment difference of 8.8% met the statistical criterion for non-inferiority (one‐sided 97.5% CI, 0.009 to 0.167; P < 0.001). In addition to meeting the criterion for non-inferiority, the proportion of responders also suggested that Probuphine was superior to sublingual buprenorphine (chi-square test P = 0.034). Relative to sublingual buprenorphine, a larger proportion of patients receiving Probuphine maintained opioid abstinence throughout six months of treatment (hazard ratio [HR] 13.8; 95% CI, 0.018 to 0.258; P = 0.03). Several post hoc sensitivity analyses (including analysis of the effect of imputing all missing urine samples as opioid positive and classifying patients who required supplemental sublingual buprenorphine as nonresponders) were performed to determine the strength of the pre-specified outcomes and the primary analysis. All sensitivity analyses were consistent with the primary efficacy results. Time to first evidence of illicit opioid use was statistically significantly lower for Probuphine relative to sublingual buprenorphine (HR 0.49; 95% CI, 0.25 to 0.97; P = 0.04). No statistically significant differences were noted between the groups in measures of craving or withdrawal.
There are several notable limitations of the PRO-814 trial. First, the efficacy outcome analyses were based on the pre-defined intention-to-treat population rather than the more conservative per-protocol population, which may have biased the results toward non-inferiority. Results for the per-protocol population were not reported. Second, concerns have been expressed regarding the expected treatment effect used to select the non-inferiority margin.50 A non-inferiority margin of 20% was selected to preserve at least 70% of the expected effect. The expected effect was the difference between the predicted responder rate of 100% with sublingual buprenorphine and 25% for placebo. However, the observed responder rate for patients receiving sublingual buprenorphine was much less than the expected 100%, and there is significant variability in the placebo response rate noted in the literature.50 Third, the generalizability of the results from PRO-814 is limited due to the demographic characteristics of the patients (i.e., the majority of patients enrolled were Caucasian, employed with at least a high school education, and with prescription opioids as the primary opioid of misuse). Enrolment was limited to stable patients who had maintained opioid abstinence on a lower dose of buprenorphine, many of whom had been receiving maintenance treatment for several years. Similar results cannot be assumed for all individuals with opioid use disorder, including those new to treatment, on high dose maintenance therapy, refractory to conventional treatment with methadone or sublingual buprenorphine, or of lower socioeconomic status. There is also some evidence that maintenance treatment may be more effective for prescription opioid misuse than for heroin misuse.52 Finally, taking into consideration that the trial enrolled patients who were stabilized for at least 90 days on sublingual buprenorphine and that Probuphine is intended to ensure compliance, the requirement of supplemental buprenorphine in a higher proportion of patients receiving Probuphine than sublingual buprenorphine, requires further investigation.
Ongoing Clinical Trial
An ongoing, open-label, crossover study is evaluating the effect of Probuphine on sleep disturbances among 15 patients with opioid use disorder who are clinically stable on 8 mg or less of Subutex or Suboxone.48 Patients will wear an actigraphy device continuously for 12 weeks to monitor activity levels and sleep patterns. Patients will continue their previously scheduled treatment regimen for the first four weeks of the study and then be switched over to Probuphine for the remaining eight weeks. Actigraphy measurements will include total time asleep, total time awake, sleep efficiency, and time mobile. The primary outcome will be the number of patients with changes in energy levels over a 90-day period. Secondary outcome measures include the number of patients with changes in sleep quality and quality of life. Changes in self-reported and clinicianreported symptoms of withdrawal will also be evaluated. Study completion is anticipated in April 2017.
Adverse Effects
The six-month safety information for Probuphine from the three published double-blind phase III trials42,43,45 as well as two unpublished open-label extension studies53,54 conducted to evaluate the safety of Probuphine for an additional six months is described in the following paragraphs. Results from the three phase III trials showed that the safety profile of Probuphine related to the buprenorphine drug substance was consistent with other approved buprenorphine drug products, which has been well characterized, and no new safety signals were identified. No deaths or serious adverse events (SAEs) requiring hospitalization were related to Probuphine. Therefore, the principal safety consideration for Probuphine is the potential for implant site adverse events related to the insertion and removal of the implants.
Phase III Double-Blind Trials
The overall implant site safety profile was comparable between treatment groups.42,43,45 Implant site adverse events decreased across studies as procedural tools, techniques, and training were optimized. The three study withdrawals for implant site adverse events (two cases of implant site pain and infection and one case of implant site pain) occurred in the first double-blind study (PRO-805). No patients withdrew for implant site–related adverse events in subsequent studies. Commonly reported implant site adverse events such as pain, pruritus, and erythema were mild and resolved without treatment. Table summarizes implant site adverse events pooled from the three phase III double-blind trials as presented in the US prescribing information for Probuphine.25
Table 2: Implant Site Adverse Events reported by ≥ 2% of Patients in Phase III Trials (Pooled)25
| Study | Main Inclusion/Exclusion Criteria | Study Details | Baseline Characteristics |
|---|---|---|---|
| Any implant site adverse event | 115 (37) | 54 (27) | 168 (33) |
| Pain | 39 (13) | 18 (9) | 57 (11) |
| Pruritus | 38 (12) | 15 (8) | 53(11) |
| Erythema | 32 (10) | 13 (7) | 45(9) |
| Hematoma | 20 (7) | 15 (8) | 35(7) |
| Hemorrage | 23 (7) | 10 (5) | 33(7) |
| Edema | 16 (5) | 5 (3) | 21(4) |
One (1.1%) case of device expulsion was reported in the sublingual buprenorphine with the placebo implant group during the PRO-814 trial.45 One (1.1%) case each of incision site complication and post-operative wound complication were reported in the Probuphine group during the PRO-814 trial.45 There was no evidence of unscheduled implant removal or attempted removal in any of the phase III trials. There were no cases of migration of implants beyond the local insertion site.
Open-label Extension Studies
Patients completing 24 weeks of treatment with Probuphine or placebo in PRO-805 were eligible to enroll in the PRO-807 open-label, re-treatment extension study.53 Sixty-two patients met eligibility criteria and received a brief induction with 12 mg to 16 mg of sublingual buprenorphine per day, in consideration of the placebo patients who were not exposed to Probuphine during PRO-805. All patients initially received four Probuphine implants and were provided sublingual buprenorphine rescue medication as clinically indicated. Six (9.7%) patients received a fifth implant (the number requiring supplemental sublingual buprenorphine was not reported). Forty-six (74%) patients completed six months of treatment.55 The incidence of adverse events was similar to PRO-805. There were no reports of SAEs. Implant site adverse events included erythema (25.8%), edema (12.9%), pruritus (19.4%), and hemorrhage (16.1%). One (1.6%) patient had an implant site adverse event that was rated as severe, but it was not described further in the poster. There was no evidence of unscheduled implant removal or attempted removal.55
PRO-811 was the second open-label re-treatment study conducted in patients completing 24 weeks of treatment in PRO-806 with either Probuphine, placebo, or sublingual buprenorphine.54 Study participants (N = 85) underwent a brief induction with 12 mg to 16 mg of sublingual buprenorphine per day before four Probuphine implants were inserted in the upper arm.56 Patients had access to a fifth implant and supplemental sublingual buprenorphine. Seventeen (20%) patients used supplemental sublingual buprenorphine and nine (11%) required a fifth implant.57 Sixty-seven (79%) patients completed six months of treatment.56 Twelve (14.1%) patients experienced implant site reactions (individual implant site adverse events were not reported in the abstract). None of the implant site events was considered an SAE or led to study discontinuation. There was no evidence of unscheduled implant removal or attempted removal.56
Cost
The manufacturer’s price for Probuphine in Canada is currently unavailable as it has not been approved.
In the US, the wholesale acquisition cost for Probuphine is priced at US $4,950 for a six-month course of treatment.58 At the wholesale acquisition cost, Probuphine is more costly than maintenance treatment with generic buprenorphine sublingual tablets (US $1,671.80) or Suboxone sublingual films (US $2,660.40) or generic buprenorphine-naloxone sublingual tablets (US $2,813.90) for six months treatment.58 On a monthly basis, Probuphine is less expensive than Vivitrol (naltrexone for extended-release injectable suspension), the only other long-acting non-oral treatment approved for opioid use disorder in the US (US $825 versus US $1,280, monthly, respectively).59 Braeburn Pharmaceuticals is exploring value-based reimbursement approaches with insurers where a rebate is offered if the overall cost of care for a patient taking Probuphine exceeds the cost of treatment for the same patients in a prior six-month period, or a comparable group of patients taking other forms of buprenorphine (or other available medications for opioid use disorder ) for a six-month period.60 A payment assistance program is also made available to ensure access to Probuphine for patients.60 It is unclear how the US cost and reimbursement options might be extrapolated to the Canadian setting.
Concurrent Developments
The following section briefly describes some of the other treatment options for opioid use disorder, in development, and that are to be administered on a weekly or a monthly basis.
RBP-6000 (Indivior PLC) is an investigational sustained-release monthly depot of buprenorphine for the treatment of opioid use disorder.61 After subcutaneous injection, the novel delivery system solidifies on contact with bodily fluids, forming a depot that releases buprenorphine continuously over one month. Positive preliminary results of a pivotal phase III clinical trial in new entrants to opioid use disorder therapy were released in August 2016.61 This was a multi-centre, randomized, double-blind, placebo-controlled study, which randomized 489 patients with moderate or severe opioid use disorder to one of two dose regimens of RBP-6000 or placebo separated by 28 days. The primary objective of this study was to assess the efficacy of monthly subcutaneous injections of RBP-6000 in two dosing regimens containing either 300 mg buprenorphine for six injections, or 300 mg for two injections followed by 100 mg buprenorphine for four injections, compared with placebo during a six-month dosing period in new entrants to medication-assisted treatment for opioid use disorder. Results showed that RBP-6000 achieved the primary outcome of the cumulative distribution function of the percentage of urine samples negative for opioids combined with self-reports negative for illicit opioid use collected from week five through week 24 (P < 0.0001 for both dosage regimens versus placebo). Safety results showed 2.8% of patients receiving RBP-6000 (both dosage regimens combined) experienced a serious treatmentemergent adverse event (TEAE) compared with 5.1% of patients on placebo. There were no related serious TEAEs across groups. Of patients receiving RBP-6000 (both dosage regimens combined), 7.2% experienced a severe TEAE compared with 4.0% of patients on placebo. Full results from the study, as well as the open-label extension to monitor safety in patients completing the double-blind trial, are expected in early 2017.
Two phase III clinical trials are being conducted to evaluate the long-term efficacy and safety of CAM2038 (Braeburn Pharmaceuticals and Camurus), two long-acting subcutaneous buprenorphine injections.62,63 CAM2038 is being developed as once-weekly and once-monthly formulations that can be titrated to cover all phases of treatment from initiation through maintenance for the treatment of opioid use disorder.62,63 Both trials are evaluating the use of CAM2038 in new entrants to treatment as well as patients who are currently receiving maintenance treatment with sublingual buprenorphine. The first randomized, double-blind, double-dummy, active-controlled phase III trial is designed to evaluate the non-inferiority of CAM2038 compared with sublingual buprenorphine-naloxone for initiation and maintenance treatment in 428 patients with moderate-to-severe opioid use disorder.64 The primary outcome measure is treatment response based on the percentage of urine samples negative for opioids combined with self-reports. Preliminary results show that CAM2038 achieved statistical non-inferiority compared with sublingual buprenorphinenaloxone for both FDA and European Medicines Agency (EMA) specified end points of responder rate (95% CI, –3.5% to 10.5%; P < 0.001) and percentage of urine samples negative for opioids (95% CI, 0.2% to 13.7%, P < 0.001).65 CAM2038 also demonstrated statistical superiority against sublingual buprenorphine-naloxone for a secondary end point of cumulative distribution function of percentage of urine samples negative for opioids combined with self-reports for weeks five to 24 (P =0.004). The overall safety profiles were comparable between the two treatment groups with few SAEs reported for CAM2038 (3.2%) and sublingual buprenorphine-naloxone (6.0%). Injection site reactions occurred in 19% of the CAM2038 patients versus 22% of those receiving buprenorphine-naloxone. Seventy-four per cent of injection site reactions were reported as mild, 26% as moderate, and none was reported as severe. A second open-label 12-month trial will assess the safety and tolerability of CAM2038 once-weekly and once-monthly in 100 patients with opioid use disorder.66 Results are anticipated in early 2017.
Vivitrol (naltrexone for extended-release injectable suspension, Alkermes Inc.) is an opioid antagonist administered as a once monthly intramuscular injection to prevent relapse, following opioid detoxification.67 Naltrexone blocks the effects of opioids without producing any opioid effects or physical tolerance. However, treatment with oral short-acting naltrexone is not commonly used in clinical practice because of the need to fully detoxify to avoid precipitating withdrawal and poor adherence to daily dosing.20 The extended-release formulation was designed to increase treatment adherence in patients with opioid use disorder, but it is available in Canada only for research purposes through Health Canada’s SAP.2 One ongoing, randomized, open-label trial is assessing the comparative effectiveness of Vivitrol versus buprenorphine-naloxone in 600 adults with opioid use disorder.67,68 It is the first large trial to evaluate the treatment outcomes of Vivitrol compared with an agonist maintenance standard of care in new entrants to opioid use disorder treatment. The primary outcome is time to opioid relapse (i.e., loss of persistent abstinence) across the 24-week treatment phase. A second ongoing randomized, open-label trial is evaluating Vivitrol versus treatment as usual with buprenorphine and psychosocial counselling in 340 youth (aged 15 to 21 years) with opioid use disorder.69 The primary outcome is opioid use at six months.
Rate of Technology Diffusion
Given the significant burden of prescription drug misuse on the Canadian health care system and the current intensified efforts to increase access to safe and effective treatment options for opioid use disorder by regulators, health care providers, and health advocates, it is anticipated that if approved, there might be significant interest in Probuphine. However, use will likely be limited to maintenance therapy in a subpopulation of patients with opioid use disorder who have achieved prolonged clinical stability on low-to-moderate doses of a transmucosal buprenorphine-containing product (i.e., doses of no more than 8 mg per day). Future research may increase the accessibility of Probuphine, should efficacy and safety be established in a broader patient population. According to the clinical expert consulted for this bulletin, one population where Probuphine could be investigated is unstable, non-treatment-seeking patients who have had repeated overdoses or are at risk of repeated overdoses. (Dr. Peter Selby, Director, Medical Education; Clinician Scientist, Addictions, Centre for Addiction and Mental Health, Toronto, ON: personal communication, 2017 Feb). Another factor that will impact the diffusion of this technology is its price and the evidence of cost-effectiveness related to other maintenance therapies for opioid use disorder.
Implementation Issues
In the US, Probuphine must be prescribed and dispensed according to the Probuphine REMS program because of the risks of surgical complications from improper insertion and removal such as implant migration, extrusion, and nerve injury.25 As part of this program, Probuphine can be prescribed and dispensed only by health care providers who are certified with the REMS program and have completed live training. In Canada, an implementation plan and risk management strategy would be discussed and determined during the New Drug Submission (NDS) review with Health Canada (Knight Therapeutics Inc., Westmount, QC: personal communication, 2016 Sep). Given that the eligible patient population will likely be limited to those with clinically stable opioid use disorder, the frequency of any single prescriber having to perform the procedure in any given setting will be low, which may negatively impact the development and maintenance of procedural competence. Although the adoption of Probuphine into clinical practice would be associated with additional costs related to the drug, training, and insertion and removal procedures, these costs may be offset if additional research provides evidence of reductions in crime and in use of health resources related to the transmission of infectious diseases and other health risks associated with opioid use disorder. Although the manufacturer is exploring value-based reimbursement programs in the US, it is unknown if there will be similar interest from Canadian payers.
Probuphine is currently not recommended for use after two six-month treatment cycles. More research is required to determine the rate and predictors of relapse after implant discontinuation and if further treatment cycles may be warranted in some patients with opioid use disorder. Although there was no evidence of attempted implant removal in the phase III trials, the potential for misuse, diversion, and accidental exposure remains a concern should the implant be intentionally removed by the patient.25 It is unclear how the requirement for supplemental therapy with sublingual buprenorphine demonstrated in the three phase III clinical trials will translate into clinical practice should therapy with Probuphine implants be adopted. In particular, if prescriptions of as-needed sublingual buprenorphine are anticipated to be routine practice, it is important to consider how this could impact the ability of this new technology to mitigate misuse and accidental pediatric exposure, particularly in patients who were previously stabilized on another maintenance therapy.50 In addition, guidance is needed for how supplemental therapy should be used, given the non-titratable nature of Probuphine.50 The US prescribing information states that alternative buprenorphine products should be considered for patients who require supplemental transmucosal buprenorphine on an ongoing basis, as this is an indication that the amount of buprenorphine delivered by Probuphine is not adequate for stable maintenance.25 Furthermore, current evidence supports the use of Probuphine only in a small subset of patients with opioid use disorder. Further studies are needed in order to determine the clinical impact and value of Probuphine in a broad and diverse population of patients with opioid use disorder, including those in remote areas. In addition, acute pain management in patients being treated with Probuphine will need to be investigated.
References
- Handford C, Kahan M, Srivastava A, Cirone S, Sanghera S, Palda V, et al. Buprenorphine/naloxone for opioid dependence: clinical practice guideline [Internet]. Toronto (ON): Centre for Addiction and Mental Health; 2011. [cited 2016 Oct 12]. Available from:http://www.cpso.on.ca/uploadedFiles/policies/guidelines/office/buprenorphine_naloxone_gdlns2011.pdf
- British Columbia Centre on Substance Use, British Columbia Ministry of Health. A guideline for the clinical management of opioid use disorder [Internet]. Coquitlam (BC): The Centre; 2016. [cited 2016 Oct 12]. Available from: http://www.vch.ca/media/Opioid-Addiction-Guideline.pdf
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th. The Association: Arlington (VA); 2013.
- Nosyk B, Anglin MD, Brissette S, Kerr T, Marsh DC, Schackman BR, et al. A call for evidence-based medical treatment of opioid dependence in the United States and Canada. Health Affairs [Internet]. 2013 [cited 2016 Aug 25];32(8):1462-9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4570728/pdf/nihms-708768.pdf
- Prescription opioids. Ottawa: Canadian Centre on Substance Abuse; 2016.
- O'Connor PG. Advances in the treatment of opioid dependence: continued progress and ongoing challenges. JAMA. 2010 Oct 13;304(14):1612-4.
- Gilson AM, Kreis PG. The burden of the nonmedical use of prescription opioid analgesics. Pain Med [Internet]. 2009 Jul [cited 2017 Jun 2];10 Suppl 2:S89-100. Available from: http://painmedicine.oxfordjournals.org/content/painmedicine/10/suppl_2/S89.full.pdf
- Government of Canada [Internet]. Ottawa (ON): Government of Canada. News release, archived - Harper Government moves forward on regulating tamper-resistant properties for prescription drugs; 2015 Jun 26 [cited 2016 Oct 19]. Available from: http://news.gc.ca/web/article-en.do?nid=992399&tp=1
- Government of Canada [Internet]. Ottawa: Government of Canada. Canadian tobacco, alcohol and drugs survey (CTADS): 2013 summary; 2015 Feb 3 [cited 2016 Oct 26]. Available from: http://healthycanadians.gc.ca/science-research-sciences-recherches/data-donnees/ctads-ectad/summary-sommaire-2013-eng.php
- Canadian Institute for Health Information, Canadian Centre on Substance Abuse. Hospitalizations and emergency department visits due to opioid poisoning in Canada [Internet]. Ottawa (ON): The Institute; 2016. [cited 2017 Jan 10]. Available from: https://secure.cihi.ca/free_products/Opioid%20Poisoning%20Report%20%20EN.pdf
- Fischer B, Argento E. Prescription opioid related misuse, harms, diversion and interventions in Canada: a review. Pain Physician. 2012 Jul;15(3 Suppl):ES191-ES203.
- Fischer B, Jones W, Murphy Y, Ialomiteanu A, Rehm J. Recent developments in prescription opioid-related dispensing and harm indicators in Ontario, Canada. Pain Physician. 2015 Jul;18(4):E659-E662.
- Death involving fentanyl in Canada, 2009-2014. Ottawa: Canadian Centre on Substance Abuse; 2015. (CCENDU bulletin).
- Prescribing and dispensing policies to address harms associated with prescription drug abuse [Internet]. Ottawa (ON): CADTH; 2015 Oct. [cited 2016 Oct 12]. (Environmental scan; no. 52). Available from: https://www.cadth.ca/sites/default/files/pdf/ES0291_Prescription_Drug_Abuse_e.pdf
- Institute for Clinical and Economic Review. Management of patients with opioid dependence: a review of clinical, delivery system, and policy options [Internet]. Boston (MA): The Institute; 2014 Jun 20. [cited 2016 Oct 12]. Available from: http://icer-review.org/wp-content/uploads/2014/04/CEPAC-Opioid-Dependence-Final-Report-For-Posting-July-211.pdf
- Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid maintenance medicines for the treatment of dependence on opioid pain medicines. Cochrane Database Syst Rev [Internet]. 2016 [cited 2016 Oct 12];(1):CD011117. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011117.pub2/full
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence (review). Cochrane Database Syst Rev [Internet]. 2014 [cited 2016 Oct 12];(2):CD002207. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD002207.pub4/epdf
- Luce J, Strike C. A cross-Canada scan of methadone maintenance treatment policy developments [Internet]. Ottawa (ON): Canadian Executive Council on Addictions; 2011. [cited 2016 Oct 14]. Available from: http://www.ceca-cect.ca/pdf/CECA%20MMT%20Policy%20Scan%20April%202011.pdf
- Buprenorphine/Naloxone versus Methadone for the treatment of opioid dependence: a review of comparative clinical effectiveness, cost-effectiveness and guidelines [Internet]. Ottawa (ON): CADTH; 2016 Sep 2. (CADTH rapid response report: summary with critical appraisal). [cited 2017 Feb 6]. Available from: https://www.cadth.ca/sites/default/files/pdf/htis/sep-2016/RD0032_Suboxone_Final.pdf
- Li X, Shorter D, Kosten TR. Buprenorphine in the treatment of opioid addiction: opportunities, challenges and strategies. Expert Opin Pharmacother. 2014;15(15):2263-75.
- Centers for Disease Control and Prevention (CDC). Emergency department visits and hospitalizations for buprenorphine ingestion by children--United States, 2010-2011. MMWR Morb Mortal Wkly Rep. 2013 Jan 25;62(3):56.
- PR Newswire [Internet]. [place unknown]: PR Newswire. News release, Braeburn Pharmaceuticals and Knight Therapeutics announce Canadian sublicense agreement for Probuphine®; 2016 Feb 1 [cited 2016 Oct 12]. Available from: http://www.prnewswire.com/news-releases/braeburn-pharmaceuticals-and-knight-therapeutics-announce-canadian-sublicense-agreement-for-probuphine-300212592.html
- Titan Pharmaceuticals [Internet]. San Francisco (CA): Titan Pharmaceuticals, Inc. Product pipeline: Probuphine® for the treatment of opioid addiction; 2016 [cited 12016 Oct 12]. Available from: http://www.titanpharm.com/pipeline/probuphine
- Titan Pharmaceuticals [Internet]. San Francisco (CA): Titan Pharmaceuticals, Inc. Proneura™; 2016 [cited 12016 Oct 12]. Available from: http://www.titanpharm.com/technology
- Probuphine: highlights of prescribing information. Princetown (NJ): Braeburn Pharmaceuticals, Inc.; 2016.
- Compton WM, Volkow ND. Improving outcomes for persons with opioid use disorders: buprenorphine implants to improve adherence and access to care. JAMA. 2016 Jul 19;316(3):277-9.
- U.S. Food & Drug Administration [Internet]. Silver Spring (MD): Food and Drug Administration. FDA news release, FDA approves first buprenorphine implant for treatment of opioid dependence; 2016 May 26 [cited 2016 Oct 12]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm503719.htm
- Webster PC. Medically induced opioid addiction reaching alarming levels. CMAJ [Internet]. 2012 Feb 21 [cited 2016 Nov 11];184(3):285-6. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3281150
- Single E. A sociodemographic profile of drug users in Canada. Ottawa (ON): HIV/AIDS Prevention and Community Action Programs of Health Canada; 2000.
- National Collaborating Centre for Mental Health. Drug misuse: opioid detoxification [Internet]. Leicester (GB): The British Psychological Society and the Royal College of Psychiatrists; 2008. [cited 2016 Oct 26]. (NICE clinical practice guideline; no. 52). Available from: https://www.nice.org.uk/guidance/cg52/evidence/drug-misuse-opioid-detoxification-full-guideline-196515037
- Barnwal P, Das S, Mondal S, Ramasamy A, Maiti T, Saha A. Probuphine® (buprenorphine implant): a promising candidate in opioid dependence. Ther Adv Psychopharmacol. 2016.
- Cushman P, Jr. Detoxification after methadone maintenance treatment. Ann N Y Acad Sci. 1981;362:217-30.
- Hubbard RL, Craddock SG, Anderson J. Overview of 5-year followup outcomes in the drug abuse treatment outcome studies (DATOS). J Subst Abuse Treat. 2003 Oct;25(3):125-34.
- Stimmel B, Goldberg J, Cohen M, Rotkopf E. Detoxification from methadone maintenance: risk factors associated with relapse to narcotic use. Ann N Y Acad Sci. 1978;311:173-80.
- College of Physicians and Surgeons of Ontario. Methadone maintenance treatment program standards and clinical guidelines [Internet]. Toronto (ON): The College; 2011. [cited 2017 Jun 2]. Available from: http://www.cpso.on.ca/uploadedFiles/members/MMT-Guidelines.pdf
- The Globe and Mail [Internet]. Toronto (ON): The Globe and Mail Inc. Canada lags behind in adopting safer drug for opioid addiction treatment; 2016 Aug 24 [cited 2016 Oct 12]. Available from: http://www.theglobeandmail.com/news/national/canada-lags-behind-in-adopting-safer-drug-for-opioid-addiction-treatment/article31526916/
- Health Canada [Internet]. Ottawa (ON): Health Canada. Methadone program; 2016 [cited 2016 Oct 13]. Available from: http://www.hc-sc.gc.ca/hc-ps/substancontrol/exemptions/methadone-eng.php
- CBC news [Internet]. Toronto (ON): Canadian Broadcasting Corporation. Ontario makes safer treatment drug widely available under strategy to battle 'growing opioid crisis'; 2016 Oct 12 [cited 2016 Oct 19]. Available from: http://www.cbc.ca/news/canada/toronto/suboxone-announcement-1.3801321
- College of Physicians & Surgeons of Alberta [Internet]. Edmonton (AB): College of Physicians & Surgeons of Alberta. Buprenorphine prescribing; 2016 [cited 2016 Oct 26]. Available from: http://www.cpsa.ca/physician-prescribing-practices/buprenorphine-prescribing/
- The College of Physicians and Surgeons of Ontario. Frequently asked questions about prescribing buprenorphine [Internet]. Toronto: The College; 2015. [cited 2016 Oct 26]. Available from: http://www.cpso.on.ca/CPSO/media/documents/Methadone/FAQs-Prescribing-Buprenorphine.pdf
- College of Physicians and Surgeons of British Columbia [Internet]. Vancouver (BC): College of Physicians and Surgeons of British Columbia. Important notice regarding Suboxone®; 2016 Jul 4 [cited 2016 Nov 4]. Available from: https://www.cpsbc.ca/important-notice-regarding-suboxone%C2%AE
- Ling W, Casadonte P, Bigelow G, Kampman KM, Patkar A, Bailey GL, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010 Oct 13;304(14):1576-83.
- Rosenthal RN, Ling W, Casadonte P, Vocci F, Bailey GL, Kampman K, et al. Buprenorphine implants for treatment of opioid dependence: randomized comparison to placebo and sublingual buprenorphine/naloxone. Addiction. 2013 Dec;108(12):2141-9.
- The Star. [Internet]. Toronto: The Star. Canada allows heroin to be prescribed in severe opioid addiction cases; 2016 Sep 8 [cited 2016 Oct 12]. Available from: https://www.thestar.com/news/canada/2016/09/08/canada-allows-heroin-to-be-prescribed-in-severe-opioid-addiction-cases.html
- Rosenthal RN, Lofwall MR, Kim S, Chen M, Beebe KL, Vocci FJ, et al. Effect of buprenorphine implants on illicit opioid use among abstinent adults with opioid dependence treated with sublingual buprenorphine: a randomized clinical trial. JAMA. 2016 Jul 19;316(3):282-90.
- Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: a systematic review. J Addict Dis. 2016;35(1):22-35.
- Mitchell SG, Gryczynski J, Schwartz RP, Myers CP, O'Grady KE, Olsen YK, et al. Changes in quality of life following buprenorphine treatment: relationship with treatment retention and illicit opioid use. J Psychoactive Drugs [Internet]. 2015 Apr [cited 2017 Feb 10];47(2):149-57. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4425232
- Kachnowski S. Probuphine Innovations for Clinical Effectiveness (PRINCE) [cited 2016 Oct 12; updated 2016 Sep 6]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine; 2000 - . Available from: https://clinicaltrials.gov/ct2/show/NCT02896660?term=probuphine+implant&rank=2 Identifier: NCT02896660.
- Basu D, Kumar V. Buprenorphine implants and opioid dependence. JAMA. 2011 Jan 19;305(3):253-4.
- U.S. Food & Drug Administration [Internet]. Silver Spring (MD): U.S. Food and Drug Administration. Briefing information for the January 12, 2016 meeting of the Psychopharmacologic Drugs Advisory Committee (PDAC); 2016 Jan 12 [cited 2016 Oct 13]. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/ucm480731.htm
- A study of adult outpatients with opioid dependence transitioned from a daily SL buprenorphine to Probuphine® subdermal implants (PRO-814). 2014 Jul 1 [cited 2017 Feb 10; updated 2015 Nov 5]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02180659 Identifier: NCT02032433.
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O'Connor PG, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med [Internet]. 2007 Apr [cited 2016 Nov 11];22(4):527-30. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1829433
- Extension to safety and efficacy of Probuphine in the treatment of opioid dependence (PRO-807). 2008 Feb 26 [cited 2016 Oct 13; updated 2014 Oct 06]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine; 2000 - . Available from: https://clinicaltrials.gov/ct2/show/NCT00630201?intr=probuphine+implant&rank=6 Identifier: NCT00630201.
- Beebe KL. Re-treatment study of Probuphine in opioid addiction. 2010 Dec 15 [cited 2016 Oct 12; updated 2014 Oct 06]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine; 2000 - . Available from: https://clinicaltrials.gov/ct2/show/NCT01262261?intr=probuphine+implant&rank=3 Identifier: NCT01262261.
- Beebe K, Ling W, Casadonte P, Rotrosen J, Yen JC, Henley SD. Safety, efficacy, and pharmacokinetics of Probuphine®, a 6-month implantable sustained-release formulation of buprenorphine, for the treatment of opioid addiction [Internet]. Poster presented at: American College of Neuropsychopharmacology 48th Annual Meeting; 2009 Dec 6-10; Hollywood (FL). [cited 2016 Oct 13]. Available from: http://c.eqcdn.com/_242cdf2269d24339daf471a9da1024e1/titanpharm/db/341/1104/pdf/ACNP-2009-Probuphine.pdf
- Beebe KL, Chavoustie S, Ling W, Sigmon S, Leiderman D, Bailey G. Buprenorphine implants for the treatment of opioid dependence: six and 12 month outcomes [abstract]. Neuropsychopharmacology. 2012;38:S266-S267. (Presented at 51st Annual Meeting of the American College of Neuropsychopharmacology, ACNP 2012 Hollywood, FL, Dec 2-6, 2012).
- Titan Pharmaceuticals [Internet]. South San Francisco (CA): Titan Pharmaceutcals. Press release, Titan Pharmaceuticals annouces positive results of six-month open-label safety retreatment study of Probuphine; 2012 Feb 9 [cited 2016 Oct 19]. Available from: http://www.titanpharm.com/news/press-releases/detail/84/titan-pharmaceuticals-announces-positive-results-of
- Buprenorphine implants (Probuphine) for opioid dependence. Med Lett Drugs Ther. 2016 Jul 18;58(1499):94-5.
- The Indiana Family and Social Services Administration. Medicaid drug spending on anti-addiction medication and opioid addiction refresher [Internet]. Indianapolis (IN): The Administration; 2015. [cited 2017 Jan 10]. Available from: https://www.in.gov/fssa/files/MAC_Presentation_20150213.pdf
- Braeburn Pharmaceuticals [Internet]. Princeton (NJ): Braeburn Pharmaceuticals. Press release, Braeburn Pharmaceuticals announces commercialization plans for Probuphine® (buprenorphine) implant, six-month treatment for opioid dependence; 2016 May 31 [cited 2016 Oct 12]. Available from: https://braeburnpharmaceuticals.com/braeburn-pharmaceuticals-announces-commercialization-plans-for-probuphine-buprenorphine-implant-six-month-treatment-for-opioid-dependence/
- Indivior [Internet]. London (GB): Indivior. Indivior announces positive top-line phase 3 pivotal study results for RBP-6000 buprenorphine monthly depot for the treatment of opioid use disorder; 2016 [cited 2016 Oct 13]. Available from: http://www.indivior.com/investor-news/rbp-6000-phase-3-top-line-results/
- Braeburn Pharmaceuticals [Internet]. Princeton (NJ): Braeburn Pharmaceuticals. Press release, Braeburn Pharmaceuticals and Camurus enroll first patient in a phase 3 efficacy trial of long-acting treatment for opioid dependence; 2015 Dec 30 [cited 2016 Oct 19]. Available from: https://braeburnpharmaceuticals.com/braeburn-pharmaceuticals-and-camurus-enroll-first-patient-in-a-phase-3-efficacy-trial-of-long-acting-treatment-for-opioid-dependence/
- Braeburn Pharmaceuticals [Internet]. Princeton (NJ): Braeburn Pharmaceuticals. Press release, Braeburn Pharmaceuticals and Camurus announce start of Phase 3 trial of long-acting buprenorphine treatments for opioid dependence; 2015 Dec 15 [cited 2016 Oct 19]. Available from: https://braeburnpharmaceuticals.com/braeburn-pharmaceuticals-and-camurus-announce-start-of-phase-3-trial-of-long-acting-buprenorphine-treatments-for-opioid-dependence/
- Clinical trial of CAM2038, long-acting subcutaneous buprenorphine injections for treatment of patients with opioid dependence. 2015 Dec 30 [cited 2016 Oct 19; updated 2016 Aug 18]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine; 2000 - . Available from: https://clinicaltrials.gov/ct2/show/NCT02651584 Identifier: NCT02651584.
- Braeburn Pharmaceuticals [Internet]. Princeton (NJ): Braeburn Pharmaceuticals. Press release, positive top-line phase 3 results for buprenorphine (CAM2038); 2016 Nov 14 [cited 2017 Jan 10]. Available from: https://braeburnpharmaceuticals.com/braeburn-pharmaceuticals-and-camurus-announce-positive-top-line-phase-3-results-for-long-acting-buprenorphine-cam2038-for-treatment-of-opioid-addiction/
- Long-term safety study of buprenorphine (CAM2038) in adult outpatients with opioid use disorder. 2016 Jan 15 [cited 2016 Oct 19; updated 2016 Jul 1]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine; 2000 - . Available from: https://clinicaltrials.gov/ct2/show/NCT02672111?term=CAM2038&rank=1 Identifier: NCT02672111.
- Lee JD, Nunes EV, Mpa PN, Bailey GL, Brigham GS, Cohen AJ, et al. NIDA Clinical Trials Network CTN-0051, extended-release naltrexone vs. buprenorphine for opioid treatment (X:BOT): study design and rationale. Contemp Clin Trials. 2016 Aug 10.
- Rotrosen J. Extended-release naltrexone vs. buprenorphine for opioid treatment. 2013 Dec 30 [cited 2016 Oct 14; updated 2016 Aug 18]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine; 2000 - . Available from: https://clinicaltrials.gov/ct2/show/NCT02032433?term=NCT02032433&rank=1 Identifier: NCT02032433.
- Mitchell SG. Extended release naltrexone for opioid-dependent youth. 2013 Apr 25 [cited 2016 Oct 14]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine; 2000 - . Available from: https://clinicaltrials.gov/ct2/show/NCT01843023?term=vivitrol&recr=Open&no_unk=Y&cond=opioid+dependence&phase=23&rank=5 Identifier: NCT01843023.
About this Document
Author: Sarah Ndegwa, Sirjana Pant, Sheri Pohar, Monika Mierzwinski-Urban.
Cite as: Buprenorphine implant for the treatment of opioid use disorder. Ottawa: CADTH; 2017 Mar. (CADTH issues in emerging health technologies; issue 153)
Acknowledgments: CADTH would like to acknowledge the contribution of Dr. Peter Selby, MBBS, FCFP, MHSc, Professor and Clinician Scientist, Departments of Family & Community Medicine and Psychiatry, and the Dalla Lana School of Public Health, University of Toronto; Centre for Addiction and Mental Health, Addictions Division, Toronto for his review of the draft version of this horizon scan report.
ISSN: 1488-6324 (online)
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policymakers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While CADTH has taken care to ensure that the information prepared by it in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. You are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Dernière mise à jour : 21 mars 2017

