Dans ce numéro :
Numéro 23 — mars 2019
(Articles en anglais seulement)
Informer les décideurs au sujet des technologies médicales en émergence
Ce numéro du Tour d’horizon des technologies de la santé comporte de brèves synthèses d’informations sur un large éventail de nouvelles technologies médicales. Parmi les sujets abordés, vous trouverez un exosquelette pour la rééducation après un AVC, un test de métabolomique axé sur l’urine pour le dépistage du cancer colorectal, des implants d’iris artificiels personnalisés pour le traitement de l’aniridie, MR-linac — un appareil qui combine le guidage par l’image et la radiothérapie du cancer, et un service de prise en charge, soutenu par la technologie, des personnes atteintes de diabète de type 2 qui fournit des recommandations automatiques de dosage d’insuline et un soutien accru au patient. Ces technologies ont été découvertes par le biais du service d’analyse prospective de l’ACMTS en tant que sujets susceptibles d’intéresser les décideurs du domaine de la santé au Canada.
- MR-linac en radiothérapie dans le traitement du cancer
- Système de guidage de l’insuline d-NAV : un nouveau mode de gestion des besoins en insuline
- CustomFlex : un iris prothétique pour les patients atteints d’aniridia
- PolypDx : analyse métabolique par l’urine pour le dépistage du cancer colorectal
- Un exosquelette robotisé pour la réadaptation de la démarche après un AVC
- Minicompilation : rapports d’analyse prospective récents de l’ACMTS et d’autres organismes
Vos commentaires
Vous avez entendu parler d’une technologie en émergence qui pourrait avoir des répercussions sur la santé au Canada, pouvez communiquer avec nous à cette adresse : [email protected]
MR-linac for Radiation Therapy for the Treatment of Cancer
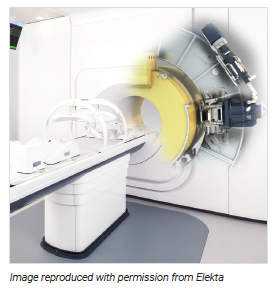 MR-linac is an emerging technology that combines a radiotherapy accelerator and a magnetic resonance imaging (MRI) scanner in a single device.1 It provides real‐time imaging of tumours at the moment of treatment and is intended to deliver radiation beams from the linear accelerator with improved targeting accuracy due to both its enhanced soft tissue visualization and monitoring during radiation delivery.1
MR-linac is an emerging technology that combines a radiotherapy accelerator and a magnetic resonance imaging (MRI) scanner in a single device.1 It provides real‐time imaging of tumours at the moment of treatment and is intended to deliver radiation beams from the linear accelerator with improved targeting accuracy due to both its enhanced soft tissue visualization and monitoring during radiation delivery.1
How It Works
Radiotherapy is an important therapeutic tool used in approximately two-thirds of all cancer patients.2 It can be used on its own or in combination with chemotherapy and/or surgery.3 Image-guided radiotherapy using CT is the current standard of care;4 however, other imaging technologies in radiation oncology are also used including megavoltage planar imaging, static kilovoltage planar imaging, and ultrasound.5
MR-linac is a hybrid device that integrates two technologies — a linear accelerator used to deliver external beam radiotherapy and an MRI scanner that guides the delivery of radiation to the tumour.1 The system enables the delivered radiation dose to be adapted to the current position and shape of the tumour and surrounding healthy tissue. It also facilitates tumour tracking for high-precision targeting.6 Theoretically, more precise targeting means that higher doses of radiation can be delivered to the cancerous tissue while minimizing damage to the surrounding tissue, which may improve clinical outcomes (increased local control, survival, decreased toxicity, improved quality of life) and reduce hospital stay compared with existing alternatives.4,6
MR-linac is the first linear accelerator system that can find tumours and treat them at the same time.7 It optimizes the visualization of images of tumours located in parts of the body where there is movement. If a tumour moves out of the targeted radiation field due to a patient’s normal internal body movement, the MR-linac automatically halts the radiation beam until the tumour returns to its original position, when radiation treatment is automatically resumed.1
There are several MR-linac systems available: the Elekta Unity that incorporates a Philips 1.5 Tesla MRI and a 7 megavolt (MV) (acceleration rate) linear accelerator,8 the MRIdian by ViewRay that integrates a 0.35 Tesla magnet and a three Cobalt-60 source for irradiation,9 and the MRIdian Linac in which the cobalt sources are replaced by a single 6 MV Linac.10 Another MR-linac, the Aurora RT radiation therapy system from MagnetTx, combines a 6 MV linear accelerator and a 0.5 Tesla MRI magnet, and has a non-clinical working prototype.11
Who Might Benefit?
It is anticipated that MR-linac will initially be used for cancers of the brain, breast, cervix, esophagus, lung, oropharynx, pancreas, prostate, and rectum.4 However, because it can potentially be used for virtually any type of cancer,12 its use is anticipated to expand.
Availability in Canada
The first MR-linac system was approved by Health Canada in 2017,13 and there are three facilities in Canada conducting research with the technology — two in Toronto and one in Edmonton.5,14
What Does It Cost?
Canadian costs are not known. In the UK, the installation of an MR-linac at one site cost £5.3M.15 At a site in the US, the installation costs amounted to US$10M.16 While the cost of an MR-linac compared to a traditional linac is not certain, it is known that they are more expensive17 and it is estimated that they may be approximately double the cost of conventional systems.12 The cost may be offset by other considerations, such as that MR-linac may provide improved outcomes or reduced costs associated with toxicity compared with other image-guided radiation therapy options.12
Initially, there may be additional staffrelated costs associated with MRI-guided radiotherapy systems which, until the procedures and tools are more mature, are operated by a team consisting of a physician, radiotherapist, medical radiation technologist, and physicist.18 In contrast, CT-guided radiotherapy only requires radiotherapists to perform the procedure.18
Current Practice
CT has been a standard of care as an imaging technique for radiation therapy simulation, planning, and image guidance.19 While CT revolutionized radiation therapy practice in the early 2000s,20 it is not the standard of care for all tumour sites and it has some limitations:
- an inability to provide high-quality, soft tissue contrast, which may make it difficult to distinguish cancerous tissue from surrounding healthy tissue15
- visualization of tumours is limited by the motion of the body (such as breathing or swallowing)17
- it can only be used before or after treatment and not during the procedure4
- it exposes patients to additional doses of radiation.4,5
Published Studies and Resources
There is a lack of published clinical studies on MR-linac because it is still mostly used for research purposes.4 A proof-of-concept study involving five patients with painful lumbar spine bone metastases was published in 2017 to determine if high-field, 1.5 Tesla MR-linac is clinically feasible in the patient population.11
Most of the ongoing studies focus on dosimetry and the technical performance and/or feasibility of MR-linac rather than on clinical outcomes. Four clinical trials are underway, including a single-arm, open-label study to measure differences between tumour targets and organs at risk volumes visualized on positron emission tomography, CT, and MRI images in lung cancer patients;21 a single-arm, open-label study to assess the technical feasibility of delivering radical radiotherapy for prostate cancer using the MR-linac;22 and two observational studies — one investigating the optimal pulse sequence parameters of MR-linac image sequences suitable for patients undergoing radiotherapy23 and the other to develop MR-linac image sequences suitable for seeing tumour/target and normal tissue at the time of radiotherapy, and to determine variations in image registrations and tissue contouring using the MR-linac images.24
Safety
There are some unique safety issues associated with MRI25 that may not be commonly understood in oncology departments,16 as some radiation oncologists may be less familiar with MRI than they are with CT. MRI safety issues relate to the projectile capabilities of metallic objects in the strong magnetic field of MRI units, as well as the necessity to screen patients for contraindications such as cardiac pacemakers and neurostimulators.16
Issues to Consider
The integration of MRI into radiotherapy practices presents several significant challenges. These challenges include processes for the planning, installation, and commissioning of the technology;19 workflow practices;26 human resources;27 education and training requirements;27 safety and quality assurance;16 and the need for collaboration between radiation oncologists, radiologists, medical physicists, pathologists, surgeons, and other staff.16,28,29
With a culture change from CT to MRI,25 significant collaboration and crosstraining between radiation therapists and medical radiation technologists will be required.16 As radiation oncology staff may be less familiar with MRI safety precautions, appropriate safety training will be important.30 Consideration may also need to be given to whether radiation therapists in the field of medical imaging require more formal qualifications and accreditation.16 As well, nuances may have to be considered such as the different requirements placed on the therapeutic use of MRI compared with the traditional diagnostic use31 (for example, compatibility with radiotherapy accessories and the higher geometric fidelity of MR images).
From a system capacity perspective, there is a concern that MR-linac may impede high-patient throughput because workflows are considered to be resourceintensive32 compared with those used for CT imaging. However, there are potential cost savings associated with MR-linac because it may be able to free up capacity by requiring fewer treatment sessions than conventional linac systems.15,33
Related Developments
MR-linac is unique in that it is the only imaging modality that is integrated into a linear accelerator.1 Other technologies available or evolving for use in informing patient positioning or image-guided therapy include ultrasound visualization, stereoscopic X-ray guidance, CT-based guidance, and continuous intra-fraction position monitoring.34
Looking Ahead
It has been predicted that, during the next ten years, MR-linac will become the standard clinical system in radiotherapy.35 It has also been suggested that it may soon enable radiation oncologists and radiation therapists to image biomarkers during treatment and adapt the treatment plan or even change the treatment objective based on real-time data.5
Author: Andra Morrison
References
- Wendler R. Merging an MRI with a linear accelerator allows greater precision in cancer treatment. 2018; https://www.mdanderson.org/publications/cancer-frontline/merging-an-mri-with-a-linear-accelerator-allows-greater-precisio.h00-159222567.html. Accessed 2019 Feb 7.
- Berkey FJ. Managing the adverse effects of radiation therapy. Am Fam Physician. 2010;82(4):381-388, 394. https://www.aafp.org/afp/2010/0815/p381.html. Accessed 2019 Feb 7.
- Gianfaldoni S, Gianfaldoni R, Wollina U, Lotti J, Tchernev G, Lotti T. An overview on radiotherapy: from its history to its current applications in dermatology. Open Access Maced J Med Sci. 2017;5(4):521-525.
- Kerkmeijer LG, Fuller CD, Verkooijen HM, et al. The MRI-Linear Accelerator Consortium: evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance, and technical development. Front Oncol. 2016;6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5061756/. Accessed 2019 Feb 7.
- Pollard JM, Wen Z, Sadagopan R, Wang J, Ibbott GS. The future of image-guided radiotherapy will be MR guided. Br J Radiol. 2017;90(1073):20160667. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5605101/. Accessed 2019 Feb 7.
- Verkooijen HM, Kerkmeijer LGW, Fuller CD, et al. R-IDEAL: a framework for systematic clincial evaluation of technical innovations in radiation oncology. Front Oncol. 2017;7:59.
- Horizon Scanning Research & Intelligence Centre. MR-LINAC image guided radiotherapy for cancer treatment. Technology ALERT. London (GB): National Institute for Health Research; 2016 Sept: http://www.io.nihr.ac.uk/wp-content/uploads/migrated/MR-LINAC-FINAL.pdf. Accessed 2019 Feb 7.
- Freeman T. Elekta Unity receives 510(k) clearance. 2018; https://physicsworld.com/a/elekta-unity-receives-510k-clearance/. Accessed 2019 Feb 7.
- Viewray Technologies. Discover MRIdian. 2018; https://viewray.com/discover-mridian/. Accessed 2019 Feb 7.
- Medgadget. FDA cleared: MRIdian Linac, the first linear accelerator and MRI in one. 2017; https://www.medgadget.com/2017/03/fda-cleared-mridian-linac-linear-accelerator-mri.html. Accessed 2019 Feb 7.
- Raaymakers BW, Jürgenliemk-Schulz IM, Bol GH, et al. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. 2017;62(23):L41-L50. https://iopscience.iop.org/article/10.1088/1361-6560/aa9517/meta. Accessed 2019 Feb 7.
- Feygelman V, Lohr F, Orton CG. The future of MRI in radiation therapy belongs to integrated MRI‐linac systems, not the standalone MRI‐Sim. Med Phys. 2017;44(3):791-794. https://aapm.onlinelibrary.wiley.com/doi/full/10.1002/mp.12090. Accessed 2019 Feb 7.
- Health Canada. Summary basis of decision - MRIdian Linac System - Health Canada. 2019; https://hpr-rps.hres.ca/reg-content/summary-basis-decision-medical-device-detailThree.php?linkID=SBD00476. Accessed 2019 Feb 7.
- CancerControl Alberta, Alberta Health Services. The whole body linac MR (Linac MRI) radiotherapy hybrid that could significantly improve cancer patient survival rates. 2018; http://www.mp.med.ualberta.ca/linac-mr/. Accessed 2019 Feb 7.
- Christie NHS Foundation Trust. Magnet delivered for revolutionary radiotherapy machine. 2016; https://www.christie.nhs.uk/about-us/news/press/four-tonne-magnet-for-revolutionary-radiotherapy-machine-that-can-see-and-treat-cancer-with-pinpoint-accuracy/. Accessed 2019 Feb 7.
- Greene J. Henry Ford offers cutting-edge MRI-guided radiation cancer therapy. 2017; https://www.crainsdetroit.com/article/20171220/news/648236/henry-ford-offers-cutting-edge-mri-guided-radiation-cancer-therapy. Accessed 2019 Feb 7.
- Zagoudis J. MRI-guided radiation therapy: a viable option for real-time visualization for adaptive radiation therapy. 2017; https://www.itnonline.com/article/mri-guided-radiation-therapy-0. Accessed 2019 Feb 7.
- van Herk M, McWilliam A, Dubec M, Faivre-Finn C, Choudhury A. MRI guided radiotherapy: a short SWOT analysis. In: Imaging & oncololgy: for imaging and therapy professionals. London (GB): Society and College of Radiographers; 2017: https://www.sor.org/system/files/article/201706/io_2017_lr.pdf. Accessed 2019 Feb 7.
- Cao Y, Tseng C-L, Balter JM, Teng F, Parmar HA, Sahgal A. MR-guided radiation therapy: transformative technology and its role in the central nervous system. Neuro Oncol. 2017;19(Suppl 2):ii16–ii29. https://academic.oup.com/neuro-oncology/article/19/suppl_2/ii16/3097614. Accessed 209 Feb 7.
- Pereira GC, Traughber M, Muzic RF. The role of imaging in radiation therapy planning: past, present, and future. Biomed Res Int. 2014:231090. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4000658/. Accessed 2019 Feb 7.
- Royal Marsden NHS Foundation Trust. NCT03658525: Prostate Radiotherapy Integrated with Simultaneous MRI (The PRISM Study) (PRISM). ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018: https://clinicaltrials.gov/ct2/show/NCT03658525. Accessed Accessed 2019 Feb 7.
- Medical College of Wisconsin. NCT03500081: Solid tumor imaging MR‐Linac (STIM study). ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018: https://clinicaltrials.gov/ct2/show/NCT03500081. Accessed 2019 Feb 7.
- Institute of Cancer Research, United Kingdom. NCT02973828: PRIMER: Development of daily online magnetic resonance imaging for magnetic resonance image guided radiotherapy. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2016: https://clinicaltrials.gov/ct2/show/NCT02973828. Accessed 2019 Feb 7.
- Christie NHS Foundation Trust. NCT03048760: Magnetic Resonance Imaging (MRI) for the delineation of Organs At Risk (OAR) and target volumes in lung cancer patients (MR-Lung). ClinicalTrials.gov. 2017. https://clinicaltrials.gov/ct2/show/NCT03048760. Accessed 2019 Feb 7.
- Pathmanathan A, van As NJ, Kerkmeijer LGW, et al. Magnetic resonance imaging-guided adaptive radiation therapy: a "game changer" for prostate treatment. Int J Radiation Oncol Biology Physics. 2018;100(2):361-373. https://www.sciencedirect.com/science/article/pii/S0360301617340269. Accessed 2019 Feb 7.
- Houssami N, Lee CI, Buist DSM, Tao D. Artificial intelligence for breast cancer screening: opportunity or hype? Breast. 2017;36:31-33.
- Erler D, D'Alimonte L, Campbell M. Opportunity is knocking: the need to responsively and responsibly integrate therapeutic MRI into radiation therapy. J Med Imag Radiation Sci. 2018;49:16-17. https://www.jmirs.org/article/S1939-8654(17)30405-8/fulltext. Accessed 2019 Feb 7.
- Chandarana H, Wang H, Tijssen RHN, Das IJ. Emerging role of MRI in radiation therapy. J Magn Reson Imaging. 2018;48(6):1468-1478.
- Lagendijk JJW, van Vulpen M, Raaymakers BW. The development of the MRI linac system for online MRI‐guided radiotherapy: a clinical update. J Intern Med. 2016;280(2):203-208. https://onlinelibrary.wiley.com/doi/full/10.1111/joim.12516. Accessed 2019 Feb 7.
- Griffis JC, Allendorfer JB, Szaflarski JP. Voxel-based Gaussian naïve Bayes classification of ischemic stroke lesions in individual T1-weighted MRI scans. J Neurosci Methods. 2016;257:97-108. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4662880/. Accessed 2019 Feb 7.
- Wong OL, Yuan J, Yu SK, Cheung KY. Image quality assessment of a 1.5T dedicated magnetic resonance-simulator for radiotherapy with a flexible radio frequency coil setting using the standard American College of Radiology magnetic resonance imaging phantom test. Quant Imaging Med Surg. 2017;7(2). http://qims.amegroups.com/article/view/13806/14717. Accessed 2019 Feb 7.
- Noble DJ, Burnet NG. The future of image-guided radiotherapy-is image everything? Br J Radiol. 2018;91(1087). https://www.birpublications.org/doi/abs/10.1259/bjr.20170894?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. Accessed 2019 Feb 7.
- Walsh M. Reducing the margin of error: MRI-guided radiotherapy. 2017; https://scienceblog.cancerresearchuk.org/2017/08/08/reducing-the-margin-of-error-mri-guided-radiotherapy/. Accessed 2019 Feb 7.
- Canadian Organization of Medical Physicists. MRI for treatment simulation: experience in the deployment of a dedicated MRI system and program expansion. 2019; https://www.comp-ocpm.ca/?lid=RX5UG-BKUT6-EMAQJ&comaction=view&id=216&key=C5XGUM358FBXTW9E3NCW. Accessed 2019 Feb 7.
- Liney GP, Whelan B, Oborn B, Barton M, Keall P. MRI-linear accelerator radiotherapy systems. Clin Oncol. 2018;30(11). https://www.clinicaloncologyonline.net/article/S0936-6555(18)30410-2/fulltext. Accessed 2019 Feb 7.
d-Nav Insulin Guidance System: A New Way to Manage Insulin Requirements
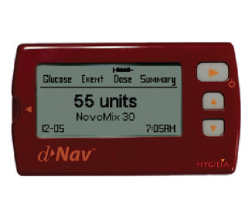 Blood sugar monitoring and insulin dose management are important elements of disease management for many people with type 2 diabetes. The d-Nav Insulin Guidance System aims to make this process easier and more effective by automating the titration of insulin dosing and providing dose guidance to inform the patient which insulin dosage to use.
Blood sugar monitoring and insulin dose management are important elements of disease management for many people with type 2 diabetes. The d-Nav Insulin Guidance System aims to make this process easier and more effective by automating the titration of insulin dosing and providing dose guidance to inform the patient which insulin dosage to use.
How It Works
The d-Nav Insulin Guidance System (Hygieia, Livonia, Michigan) leverages proprietary technology to provide personalized guidance prior to each insulin injection.1 The guidance is provided through an integrated, hand-held device that has a built-in glucose sensor to measure blood glucose levels, or via the d-Nav phone app using glucose data collected by any other available monitoring method from third parties (e.g., continuous glucose monitoring).1,2 In both cases, the software automatically monitors and analyzes glucose levels and automatically titrates the individual’s dosage of insulin based on patterns in their overall blood glucose readings.1,3 At a minimum, this adjustment in insulin dosing is made weekly.4 The blood glucose measurements and corresponding insulin requirements are stored in an online cloud, where they can be accessed and reviewed by dedicated nurses who support d-Nav users.1 These nurses communicate with participants in person or by phone to provide them with individualized support to help manage their diabetes.1
Appropriate insulin replacement can help prevent complications of diabetes and potentially reduce costs to both the patient and the health care system.2 Typically, people with diabetes visit their doctor’s offices occasionally to check on the appropriateness of their insulin dose based on blood glucose trends and to determine if adjustments are needed.2 The intention of the d-Nav system is to provide regular feedback to people with diabetes regarding the appropriate insulin dose required for each injection and to automatically adjust the insulin dose, as needed.2
Who Might Benefit?
The d-Nav system is intended for use by any person with type 2 diabetes who requires insulin.1 In 2018, an estimated 3.5 million Canadians lived with diagnosed diabetes.5 An estimated 11.4% of the Canadian population will be diagnosed with diabetes by 2025.6 Based on 2009 to 2014 US data, the percentage of people with type 2 diabetes treated with insulin was 22.2%.7 No Canadian data on the proportion of insulin use was identified.
Availability in Canada
The d-Nav system is not currently licensed for use or available for sale in Canada and there is no estimated time of entry into the Canadian market available (J. Daniel Prewitt, Chief Revenue Officer, Hygieia, Livonia, MI: personal communication, 2018 Nov 21).
The d-Nav system received US FDA 510(k) clearance in February of 2019.8 In the US, the d-Nav system is currently available in Michigan for some people with type 2 diabetes through an agreement between the manufacturer and Blue Cross Blue Shield.2 The d-Nav system has been in use by the South Eastern Health and Social Care Trust in Northern Ireland since 2012 through a partnership with the manufacturer1 and has CE marking in Europe.9
What Does It Cost?
No information on the costs of purchasing and operating the d-Nav system was identified. The manufacturer has not yet established the Canadian cost of the technology (J. Daniel Prewitt: personal communication, 2018 Nov).
One cost-effectiveness analysis was identified examining the use of the d-Nav system for people with diabetes who were at high risk of neuropathic foot ulcers within the UK NHS.10 In this study, the cost of the d-Nav system (based on 2013-2014 prices) was comprised of an installation fee (£102.17) and a weekly per-patient service fee that decreased, depending on the number of users (£44.96 to £33.72).10
The manufacturer of the device suggests that cost savings may be gained through a reduction in diabetes-related complications and hospitalizations, a reduction in outpatient clinic costs, and a reduction in the cost of diabetes testing supplies and glucose-management drugs.2
Current Practice
The Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada outlines current practices for the diagnosis and management of diabetes.11 They recommend that people with diabetes have their glycated hemoglobin (A1C) levels measured every three months when they are having glucose control issues or when making changes to their methods of diabetes management.11 Monitoring blood glucose levels using methods such as self-monitoring of blood glucose, flash glucose monitoring, continuous glucose monitoring, and checking A1C levels is recommended as the best methods of gathering information to assess an individual’s glucose control.11 Individual targets are determined in consultation with the patient’s health care team. The guideline also recommends collaborative and supportive environments to help facilitate the successful self-management of diabetes.11 Educational materials and approaches are recommended to be customized based on the individual and their risk factors and life situations.11
Published Studies and Resources
One randomized controlled trial (2018) of the d-Nav system and specialist support was undertaken for 181 people with type 2 diabetes whose condition was being managed suboptimally with insulin.4 Participants using the d-Nav system were compared with participants who received only specialist support.4 One prospective before-and-after study of the d-Nav system (two publications, 2012) was undertaken for 46 people in the US with type 1 or type 2 diabetes.12,13
The studies investigated clinical outcomes including the fraction of dose adjustments made by the d-Nav system without intervention from the study team,4,12,13 A1C levels,4,12,13 frequency of hypoglycemic events,4,12,13 and patient comfort and satisfaction.4
In addition, three conference abstracts3,14,15 and one poster presentation were identified.16 One of the abstracts3 appears to be related to the previously mentioned randomized trial. The other conference abstracts may be related to otherwise unpublished work.14,15 The poster presentation outlines the d-Nav pilot project in Ireland.16
The manufacturer noted that there are no ongoing or additional planned clinical trials (J. Daniel Prewitt: personal communication, 2018 Nov).
Safety
None of the identified studies or resources reported on potential safety issues related to the technology.
Issues to Consider
Health care providers may be challenged to process and respond in a timely manner to the large amounts of information produced through cloud-based, remote disease management systems.17 The requirement for monitoring infrastructure and training providers in supporting patients using the technology may also impact the feasibility of use.18
Related Developments
In October of 2018, Health Canada approved a hybrid, closed-loop insulin pump that automatically and continuously adjusts insulin delivery based on continuous glucose monitor readings.19 Other similar devices are expected to be released in 2019.
Other technologies that support patients with diabetes using insulin are currently on the market or in development. However, unlike d-Nav, none of these technologies provides automatic insulin dose recommendations for the patient.
The types of technologies available include mobile applications (apps) that allow people with diabetes to easily enter their blood glucose levels, insulin pump information, activity, nutrition, and lifestyle data into the app in a format that can be shared electronically with their care providers.20,21 Additionally, there are tracking apps that are paired with glucose testing devices that automatically transfer blood glucose measurements to the app,22,23 and some also facilitate communication with health care professionals to discuss test results and diabetes management through the app itself.24-26 Apps exist that are more focused on improving the communication between patient and physician to address gaps in current treatment.27 A clinical decision support software is available to help make recommendations for the management of in-patient insulin dosage in the hospital setting.28
Looking Ahead
There are a number of mobile applications currently available that intend to help people with diabetes monitor and manage their condition. They aim to support self-management and decentralize care; however, few have been proven clinically effective for long-term glycemic control and the management of A1C levels.9 In order for these applications to be more widely used, longer-term clinical trials are required to adequately demonstrate their benefits for people with diabetes.9
Author: Michelle Clark
References
- Hygieia. 2018; http://hygieia.com/. Accessed 2018 Dec 10.
- Hodish I. Insulin therapy for type 2 diabetes - are we there yet? The d-Nav story. Clin Diabetes Endocrinol. 2018;4:8.
- Hodish I, Bisgaier SG, Unger E, Austin MM. Cost savings associated with digitally enhanced insulin therapy [abstract]. Diabetes. 2018;67(Suppl 1):A355-A356. Presented at: Scientific Sessions of the American Diabetes Association; 2019 Jun; San Francisco, CA.
- Hodish I, Bergenstal RM, Johnson ML, Passi RA, Bhargava A, Young N, et al. Digitally enhanced insulin therapy-a multicenter clinical trial [abstract]. Diabetes. 2018;67(Suppl 1):A95. Presented at: Scientific Sessions of the American Diabetes Association; 2019 Jun; San Francisco, CA.
- Diabetes in Canada. Ottawa (ON): Diabetes Canada; 2018: https://www.diabetes.ca/getmedia/6960f8d5-0869-4233-8ac2-6c669dae7c59/2018-Backgrounder-Canada_KH_AB_KB-edited-13-March-2018_2.pdf.aspx. Accessed 2019 Jan 21.
- 2015 Report on diabetes: driving change. Toronto (ON): Canadian Diabetes Association (CDA); 2015: https://www.diabetes.ca/publications-newsletters/advocacy-reports/2015-report-on-diabetes-driving-change. Accessed 2018 Dec 10.
- Basu S, Yudkin JS, Kehlenbrink S, Davies JI, Wild SH, Lipska KJ, et al. Estimation of global insulin use for type 2 diabetes, 2018–30: a microsimulation analysis. Lancet Diabetes Endocrinol. 2019;7(1):25-33.
- D-Nav System; 510(k) no. K181916. 510(k) premarket notification 2019; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K181916. Accessed 2019 Feb 14.
- Drincic A, Prahalad P, Greenwood D, Klonoff DC. Evidence-based mobile medical applications in diabetes. Endocrinol Metab Clin North Am. 2016;45(4):943-965.
- Green W, Taylor M. Cost-effectiveness analysis of d-Nav for people with diabetes at high risk of neuropathic foot ulcers. Diabetes Ther. 2016;7(3):511-525.
- Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2018;42(Suppl 1).
- Bergenstal RM, Bashan E, McShane M, Johnson M, Hodish I. Can a tool that automates insulin titration be a key to diabetes management? Diabetes Technol Ther. 2012;14(8):675-682.
- Bergenstal RM, Bashan E, McShane M, Johnson M, Hodish I. Effective diabetes management via new affordable patient-friendly tool [abstract]. J Diabetes Sci Technol. 2012;6(2):A16. Presented at: Annual Diabetes Technology Meeting; 2011 Oct; San Francisco, CA.
- Hodish I, Johnson M, Bashan E, Kruger DF, Bhargava A, Bergenstal RM. Automated frequent insulin dosage titrations to enhance therapy effectiveness; lessons from the d-Nav insulin guidance [abstract]. Diabetologia. 2017;60(1 Suppl 1):S303. Presented at: Annual Meeting of the European Association for the Study of Diabetes; 2017 Sep; Lisbon, PT.
- Bi Y, Donnelly R, Heald AH, Harper R. Diabetes Insulin Guidance System (DIGSTM): a real-world evaluation of a novel assistive technology (d-NavTM) to optimise glycaemic control in those with diabetes requiring insulin therapy [abstract]. Diabet Med. 2015;32(S1):196. Presented at: Diabetes UK Professional Conference; 2015 Mar; London, UK.
- Harper R, Donnelly R, Hodish I. Insulin therapy transformation in Northern Ireland [abstract]. Diabetes. 2016;65(Suppl 1):A320. Presented at: Scientific Sessions of the American Diabetes Association, 2016 Jun; New Orleans, LA.
- Greenwood DA, Gee PM, Fatkin KJ, Peeples M. A systematic review of reviews evaluating technology-enabled diabetes self-management education and support. J Diabetes Sci Technol. 2017;11(5):1015-1027.
- Vegesna A, Tran M, Angelaccio M, Arcona S. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health. 2017;23(1):3-17.
- BC Diabetes. 2018; https://www.bcdiabetes.ca/health-canada-approves-insulin-pump-with-partial-artificial-pancreas-capability/. Accessed 2018 Dec 20.
- Glooko. 2018; https://www.glooko.com/. Accessed 2018 Dec 20.
- Bant. 2016; http://www.bantapp.com/. Accessed 2018 Dec 20.
- Dario. 2019; https://mydario.com/. Accessed 2018 Dec 20.
- GlucoMe digital diabetes care platform. 2019; https://www.glucome.com/. Accessed 2018 Dec 20.
- Onduo. 2019; https://onduo.com/. Accessed 2018 Dec 20.
- One Drop announces regulatory approval and launch in Canada [news release]. Chicago (IL): Cision Ltd; 2017: https://www.newswire.ca/news-releases/one-drop-announces-regulatory-approval-and-launch-in-canada-655631573.html.
- Agamatrix. 2019; https://agamatrix.com/diabetes-apps/. Accessed 2018 Dec 20.
- Rimidi. 2019; https://rimidi.com/. Accessed 2018 Dec 20.
- EndoTool glucose management system. Charlotte (NC): Monarch Medical Technologies; 2019: https://monarchmedtech.com/glucose-management-system/.
CustomFlex ARTIFICIALIRIS: An Iris Prosthesis for People with Aniridia
 Aniridia is an eye disorder whereby a person is missing part or all of the coloured part of the eye (the iris).1,2 A new device — a flexible, customizable iris implant recently approved by the US FDA — may help improve vision and cosmetic issues in people living with the condition.
Aniridia is an eye disorder whereby a person is missing part or all of the coloured part of the eye (the iris).1,2 A new device — a flexible, customizable iris implant recently approved by the US FDA — may help improve vision and cosmetic issues in people living with the condition.
How It Works
CustomFlex ARTIFICIALIRIS (HumanOptics AG, Erlangen, Germany)3 is a prosthetic iris originally developed by Dr. Schmidt Intraocularlinsen GmbH and is marketed under the name ARTIFICIALIRIS in Europe.4,5 It is made of a foldable, biocompatible silicone material that can be inserted into the eye through a 2.8 mm to 7.0 mm incision.6,7 The device is 12.8 mm in diameter, with a fixed-pupil aperture of 3.35 mm.7 It is available in two models: one with embedded fibre mesh (to add strength if sutured in place) and a more flexible non-mesh version.6,8,9
Each device is custom-painted, by hand, based on a photograph of the patient’s other iris or, in cases of complete aniridia, the colour of a photograph chosen by the patient.6,8-10 The back side is a smooth, opaque black.7
There are various surgical techniques for implanting the devices.7 Each technique involves a common basic preparation:
- photographing the remaining iris (the iris of the opposite eye)
- selecting the device model appropriate for the technique to be used
- implanting the device into the ciliary sulcus (the area behind the iris) using forceps or an injector system.
Who Might Benefit?
Defects in the iris can occur at birth or can be acquired later in life (from trauma to the eye, for example).2,8 In either form, aniridia may affect only a portion of the iris or the iris as a whole.2 As a congenital condition, aniridia affects an estimated one in 50,000 to 100,000 births worldwide.1 People born with aniridia are often affected by other conditions of the eye; in particular, glaucoma and cataracts.11 Vision in people with aniridia can be very good or very poor, and many people with aniridia have large pupils, making them sensitive to light.11
Studies of the CustomFlex ARTIFICIALIRIS have included people with birth-related partial or complete aniridia, or from aniridia caused by other conditions or trauma.6 Because the device is intended to be placed in front of an intraocular lens,12 people receiving the implant must either:
- already have an intraocular lens implant
- have no eye lens at all
- be eligible to have a crystalline (natural) lens removed and replaced with an intraocular lens.6
The device is not intended for use in people with a variety of associated conditions such as chronic untreated glaucoma, uveitis, untreated retinal detachment, or types of retinopathy.6 It is not intended to be used to change the colour of a person’s eye.3
In the US, the FDA has approved the device for adults and children with full or partial aniridia caused by congenital aniridia or iris damage from other causes or conditions.6 It was given Breakthrough Devices Designation for meeting a need in a population of patients without other suitable treatment options.6
Availability in Canada
CustomFlex ARTIFICIALIRIS is not yet available in Canada. It was approved for use in the US in 20186 and in Europe in 2011.9,13
What Does It Cost?
No information on the cost of CustomFlex ARTIFICIALIRIS was identified.
Published Studies and Resources
Regular eye examinations are recommended for people with aniridia to monitor changes in vision and detect glaucoma.11,14 Options to treat aniridia are limited and include tinted glasses, iris reconstruction surgery, coloured contact lenses, and corneal tattooing.6
What is the Evidence?
Two studies of the CustomFlex ARTIFICIALIRIS were identified in patients with full or partial iris defects.9,15 This includes one multi-centre, prospective, non-randomized, open-label, uncontrolled clinical trial (n = 447 eyes) currently ongoing in the US,15 with results available in FDA documentation,6 and one 2016 single-centre, prospective, uncontrolled, before-and-after study (n = 32 eyes) conducted in Germany.9
Outcomes reported by authors include:
- best-corrected visual acuity9
- intraocular pressure9
- light sensitivity and glare6,9
- quality of life6
- satisfaction with the cosmetic appearance of the implant6,9
- safety.6
Conference abstracts, case series, and case studies were not included. CADTH completed a Rapid Response on artificial iris prostheses in 2016.16
Safety
The US FDA’s summary decision lists possible adverse events related to the device or the surgical procedure.6 These include worsening vision or photosensitivity, elevated intraocular pressure, infection or inflammation, incorrect device positioning, device movement, and the need for subsequent procedures.6 Device-related, surgery-related, and intraocular lens-related adverse events were all reported in the studies we identified.6,9
The device is unsafe for use in an MRI,6 which may also be an important consideration when selecting patients for the procedure.
Issues to Consider
Implanting the device is a specialized and technical procedure7 but no literature was identified discussing accessibility of the procedure for patients living long distances from centres offering the intervention. According to the manufacturer, clinicians wishing to use the device must complete an online training course.3
Patient-important outcomes — such as satisfaction with the cosmetic appearance of the device and functional outcomes — were reported outcomes in the studies identified.6,9 However, using prosthetic iris implants for the purpose of cosmetic improvement may need to be weighed against potential intraoperative and post-operative complications.2
Related Developments
People with aniridia often have other eye conditions that require surgery.1,11 Because of this, the feasibility of conducting pars plana vitrectomy (a procedure to remove the fluid from within the eye to perform other types of repairs) through the pupil of a CustomFlex ARTIFICIALIRIS was investigated.17 Surgeons have also described implanting the device into phakic eyes (eyes with a lens implanted without the removal of the natural lens)18-20 and other emerging approaches to surgical implantation are also being investigated.5,21-23
Other iris replacements and iris implants for the treatment of aniridia exist10,24-26 and have been used in Europe for more than a decade.12 We did not identify any studies comparing these devices to the CustomFlex ARTIFICIALIRIS. Treating aniridia with an artificial cornea implant has also been described.27
Looking Ahead
A limitation of the CustomFlex ARTIFICIALIRIS is its fixed pupil.10 Future approaches to managing aniridia could involve using materials that respond to light.28-31 Researchers are currently investigating self-dimming contact lenses28 and iris implants that can change the size of the pupil in response to light.29-31
Author: Jeff Mason
References
- U.S. National Library of Medicine. Aniridia. Genetic home reference. 2018; https://ghr.nlm.nih.gov/condition/aniridia#inheritance, 2018 Dec 3.
- Srinivasan S, Ting DSJ, Snyder ME, Prasad S, Koch H-R. Prosthetic iris devices. Can J Ophthalmol. 2014;49(1):6-17.
- Human Optics AG. ARTIFICIALIris. 2018; https://www.humanoptics.com/en/physicians/artificialiris/. Accessed 2018 Dec 10.
- Koch KR, Heindl LM, Cursiefen C, Koch HR. Artificial iris devices: benefits, limitations, and management of complications. J Cataract Refract Surg. 2014;40(3):376-382.
- Doroodgar F, Jabbarvand M, Niazi F, Niazi S, Sanginabadi A. Implantation of ArtificialIris, a CustomFlex irisprosthesis, in a trauma patient with an Artisan lens: A case report and review. Medicine (Baltimore). 2017;96(45):e8405.
- Summary of Safety and Effectiveness Data (SSED): CustomFlex Artificial Iris. (PMA P170039). Silver Spring (MD): U.S. Food and Drug Administration; 2018: https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170039B.pdf. Accessed 2018 Dec 10.
- Mayer C, Tandogan T, Hoffmann AE, Khoramnia R. Artificial iris implantation in various iris defects and lens conditions. J Cataract Refract Surg. 2017;43(6):724-731.
- Rickmann A, Szurman P, Januschowski K, Waizel M, Spitzer MS, Boden KT, et al. Long-term results after artificial iris implantation in patients with aniridia. Graefes Arch Clin Exp Ophthalmol. 2016;254(7):1419-1424.
- Mayer CS, Reznicek L, Hoffmann AE. Pupillary reconstruction and outcome after artificial iris implantation. Ophthalmology. 2016;123(5):1011-1018.
- Spitzer MS, Nessmann A, Wagner J, Yoeruek E, Bartz-Schmidt KU, Szurman P, et al. Customized humanoptics silicone iris prosthesis in eyes with posttraumatic iris loss: Outcomes and complications. Acta Ophthalmol (Oxf). 2016;94(3):301-306.
- American Association for Pediatric Ophthalmology and Strabismus. Aniridia. 2016; https://aapos.org/terms/conditions/26. Accessed 2018 Dec 10.
- Doran M. Iris implants advance—but face continuing challenges. EyeNet Magazine. San Francisco (CA): American Academy of Ophthalmology; 2013: https://www.aao.org/eyenet/article/iris-implants-advance-face-continuing-challenges.
- Mayer CS, Laubichler AE, Khoramnia R, Tandogan T, Prahs P, Zapp D, et al. Challenges and complication management in novel artificial iris implantation. J Ophthalmol. 2018;2018:3262068.
- Moosajee M, Hingorani M, Moore A. PAX6-related aniridia. (GeneReviews®). Vol 2018. Seattle (WA): University of Washington; 2003: https://www.ncbi.nlm.nih.gov/books/NBK1360/. Accessed 2018 Oct 18.
- Clinical Research Consultants Inc. NCT01860612: Safety and effectiveness of the CustomFlex artificial iris prosthesis for the treatment of iris defects. Clinicaltrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018: https://www.clinicaltrials.gov/ct2/show/NCT01860612?term=NCT01860612&rank=1. Accessed 2018 Dec 10.
- Artificial iris prosthesis for aniridia: Clinical effectiveness. (Rapid response report: summary of abstracts). Ottawa (ON): CADTH; 2016: https://cadth.ca/artificial-iris-prosthesis-aniridia-clinical-effectiveness. Accessed 2019 Jan 3.
- Toygar O, Snyder ME, Riemann CD. Pars plana vitrectomy through a custom flexible iris prosthesis. Retina. 2016;36(8):1474-1479.
- Tassignon MJBR. Reply: Phakic implantation of flexible iris prosthesis. J Cataract Refract Surg. 2012;38(12):2209.
- Snyder ME, Perez MA. Phakic implantation of flexible iris prosthesis. J Cataract Refract Surg. 2012;38(12):2208-2209; author reply 2209.
- Magnus J, Trau R, Mathysen DG, Tassignon MJ. Safety of an artificial iris in a phakic eye. J Cataract Refract Surg. 2012;38(6):1097-1100.
- Yoeruek E, Bartz-Schmidt KU. A new knotless technique for combined transscleral fixation of artificial iris, posterior chamber intraocular lens, and penetrating keratoplasty. Eye. 2018;12:12.
- Ziaei M, Kim BZ, Hadden PW, McGhee CN. Battle-axe fold: Surgical technique for in-the-bag implantation of an artificial iris implant. Clin Experiment Ophthalmol. 2017;45(8):831-834.
- Spitzer MS, Yoeruek E, Leitritz MA, Szurman P, Bartz-Schmidt KU. A new technique for treating posttraumatic aniridia with aphakia: First results of haptic fixation of a foldable intraocular lens on a foldable and custom-tailored iris prosthesis. Arch Ophthalmol. 2012;130(6):771-775.
- Morcher GmbH. Aniridia implants. Morcher ophthalmic implants 2018; http://www.morcher.com/nc/en/produkte/aniridia-implants.html. Accessed 2018 Dec 10.
- Ophtec. 311 Aniridia Lens II. 2018; https://www.ophtec.com/products/trauma-surgery/implants/311-aniridia-lens-ii. Accessed 2018 Dec 10.
- Ophtec. Iris prosthetic system. 2018; https://www.ophtec.com/products/trauma-surgery/implants/iris-prosthetic-system. Accessed 2018 Dec 10.
- Rixen JJ, Cohen AW, Kitzmann AS, Wagoner MD, Goins KM. Treatment of aniridia with Boston type I keratoprosthesis. Cornea. 2013;32(7):947-950.
- Shareef F, Szlachta D, Contreras G, Chen A, Azar DT, Cho M. Designing a photo-responsive contact lens. Investig Ophthalmol Vis Sci. 2015;56 (7):6102.
- Shareef FJ, Sun S, Kotecha M, Kassem I, Azar D, Cho M. Engineering a light-attenuating artificial iris. Invest Ophthalmol Vis Sci. 2016;57(4):2195-2202.
- Zeng H, Wani OM, Wasylczyk P, Kaczmarek R, Priimagi A. Self-regulating iris based on light-actuated liquid crystal elastomer. Adv Mater. 2017;29(30).
- Na JH, Park SC, Sohn Y, Lee SD. Realizing the concept of a scalable artificial iris with self-regulating capability by reversible photoreaction of spiropyran dyes. Biomaterials. 2013;34(13):3159-3164.
PolypDx: A Urine-Based Metabolomic Test for Colorectal Cancer Screening
 Incidence and mortality of colorectal cancer can be reduced with early detection, but adequate participation in screening programs remains a challenge.1 PolypDx is a urine-based metabolomic test for detecting and preventing colorectal cancer. It aims to offer ease and accessibility of screening in addition to the current fecal occult blood test options.2,3
Incidence and mortality of colorectal cancer can be reduced with early detection, but adequate participation in screening programs remains a challenge.1 PolypDx is a urine-based metabolomic test for detecting and preventing colorectal cancer. It aims to offer ease and accessibility of screening in addition to the current fecal occult blood test options.2,3
How It Works
Metabolomics is the identification and quantification of small molecular weight chemicals generated by metabolism.4 PolypDx (Metabolomic Technologies Inc., Edmonton, Alberta) is a urine-based metabolomic screening test for detecting adenomatous polyps of the large intestine, the precursor to colorectal cancer (CRC).2 It is intended for individuals at average-to-moderate risk of CRC.3
PolypDx was developed through the metabolic profiling of 685 urine samples at the University of Alberta using nuclear magnetic resonance (NMR) technology.2 PolypDx uses liquid chromatography–mass spectrometry (LC-MS) technology to measure the urine concentration of three key urinary metabolites: succinic acid, carnitine, and ascorbic acid.2 The urine concentration of these three key urinary metabolites, along with clinical features including age, sex, and smoking history, are interpreted by a machine-learning algorithm to determine the probability of an adenomatous polyp being present.2 The test results are either positive or negative for adenoma, with positive results requiring a follow-up and a colonoscopy at the care provider’s discretion.2
Who Might Benefit?
In Canada, CRC is the third most common cause of cancer-related mortality in females and the second most common in males, with lifetime probabilities of CRC mortality of 3.1% for women and 3.5% for men.5 In 2015, approximately 25,100 Canadians were newly diagnosed with CRC (49 in every 100,000 Canadians).5
Individuals who are at risk of CRC could potentially benefit from the PolypDx technology. This includes individuals older than 50 years old — as the incidence of CRC increases with age — and those with a family history of CRC.5,6
Availability in Canada
PolypDx is currently unavailable in Canada except in research settings (Dr. Lu Deng, Senior Scientist and Director of Business Development, Metabolomic Technologies Inc., Edmonton, AB: personal communication, 2018 Nov 23). PolypDx is currently available in the US as a laboratory-developed test through Clinical Laboratory Improvement Amendments (CLIA) certified laboratories.2
What Does It Cost?
In the US, the commercial price of PolypDx is US$399 per test (Dr. Lu Deng: personal communication, 2018 Nov). In the 2019 clinical laboratory fee schedule published by the Centers for Medicare & Medicaid Services, PolypDx is priced at US$200 by its primary processing Medicare administrative contractors, Novitas Solutions.7
In Canada, the price of PolypDx is yet to be determined (Dr. Lu Deng: personal communication, 2018 Nov).
Current Practice
Established non-invasive tests, such as fecal occult blood tests (FOBT), are the main components of most CRC screening programs, which identify individuals who should receive a colonoscopy.8 These current non-invasive FOBT tests have high specificity (94.2 to 99%) but low sensitivity (2.6 to 17.6%), especially for the detection of CRC precursors like adenomas.2,4 A colonoscopy is the gold standard for the detection and removal of pre-cancer and cancerous tissues.4 However, barriers to consider are that it is invasive, costly, and inconvenient for patients.4
The 2016 guideline published by the Canadian Task Force on Preventive Health Care recommends screening adults aged 50 to 74 years for CRC with FOBT (either a guaiac fecal occult blood test or a fecal immunochemical test) every two years or flexible sigmoidoscopy (examination of the lower portion of the colon)9 every 10 years.6
Published Studies and Resources
Three diagnostic test accuracy studies of PolypDx in patients enrolled in a CRC screening program were identified.2,8,10 These include two validation studies — one conducted in Canada (n = 633)10 and one in China (n = 661).8 The studies compared an NMR-based metabolomics test — a precursor to PolypDx technology — with commercially available fecal-based tests for their ability to detect adenomas based on a reference standard of colonoscopy.8,10 The third validation study,2 conducted in Canada (n = 685), compared PolypDx (both NMR and LC-MS-based) and commercially available fecal-based tests with a reference standard of colonoscopy.2 The identified studies report on diagnostic accuracy outcomes such as sensitivity and specificity.8,10
In addition, one systematic review4 was identified that included 41 studies (including the three diagnostic test accuracy validation studies listed previously)2,8,10 investigating the diagnostic performance of metabolomic biomarkers in blood, urine, and feces for the early detection of colorectal neoplasms.
Safety
While no published information was identified on the safety of the PolypDx technology, like many screening tests, CRC screening technologies are associated with a potential risk of overdiagnosis and false-positive test results.11 Overdiagnosis applies to the proportion of cases that may not have progressed to symptom presentation if not detected via screening.11 As these cases are not distinguishable from those with poor prognosis, patients may be subjected to the adverse effects of cancer therapy with unclear benefit.
Issues to Consider
Generally, compared to blood serum samples, urine samples are more susceptible to lifestyle factors such as diet and exercise, which can affect urinary biomarker composition and affect test results.4
PolypDx uses a machine-learning algorithm to determine the probability of an adenomatous polyp being present.2 The ability of machine-learning algorithms to determine the likelihood of a polyp being present depends on the amount and quality of data available for training.12
To date, the studies have been done in individuals who are enrolled to receive a colonoscopy, so the screening and diagnostic performance in general asymptomatic populations that may be at risk of CRC is unclear.2,4,8,10
PolypDx may increase access to screening in rural and remote settings when compared with colonoscopy, and may provide an alternative to FOBT. The mass spectrometers required for PolypDx are commonly available in clinical laboratory settings, whereas a colonoscopy requires in-person testing.2 Urine samples can be easily collected and sent to central labs for analysis, negating the need to travel.
Related Developments
Metabolomic Technologies Inc. is working with researchers at the University of Alberta, Memorial Sloan Kettering Cancer Center, and Obafemi Awolowo University on a point-of-care, real-time, urine metabolomics test for the diagnosis of polyps and cancer.13 The test aims to incorporate biomarkers for the diagnosis of CRC into the current biomarker panel for PolypDx (Dr. Lu Deng: personal communication, 2018 Dec).
A number of metabolomic screening tests for CRC are being developed with dried blood spot-based and plasma-based testing.4
The C-Scan Cap system developed by Check-Cap is an orally ingested, X-ray-based imaging that scans and records the structure of the colon as it passes through the digestive tract.14
Looking Ahead
There is significant interest in combining machine-learning algorithms and diagnostic tools.15 The introduction of PolypDx into clinical practice may offer more insight into the application of machine-learning methodology in the development of screening and diagnostic tools.
As PolypDx is currently unavailable in Canada, the impact on health care human resources and financial resources for CRC screening in the Canadian health care system is unclear. The Canadian Assessment of PolypDx (CAP) project was announced in October 2018.16 It is intended to assess a new CRC screening program using PolypDx in 3,000 patients, in five regions throughout Alberta.16 The results of the new screening program will be assessed by a national working group that consists of key provincial and national leaders in CRC screening.16
Author: Ke Xin Li
References
- Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014;106(4):dju032.
- Deng L, Chang D, Foshaug RR, et al. Development and validation of a high-throughput mass spectrometry based urine metabolomic test for the detection of colonic adenomatous polyps. Metabolites. 2017;7(3):22.
- PolypDx™ for healthcare professionals. Edmonton (AB): Metabolomic Technologies Inc; 2019: https://polypdx.com/for-healthcare-providers. Accessed 2019 Jan 25.
- Erben V, Bhardwaj M, Schrotz-King P, Brenner H. Metabolomics biomarkers for detection of colorectal neoplasms: a systematic review. Cancers (Basel). 2018;10(8):27.
- Canadian Cancer Society's Advisory Committee on Cancer Statistics. Canadian cancer statistics 2015. Toronto (ON): Canadian Cancer Society; 2015: www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2015-EN.pdf. Accessed 2019 Jan 25.
- Bacchus CM, Dunfield L, Gorber SC, et al. Recommendations on screening for colorectal cancer in primary care. CMAJ. 2016;188(5):340-348.
- 2018 Clinical diagnostic laboratory fee schedule. CLFS gapfill final determinations. Baltimore (MD): Centers for Medicare & Medicaid Services; 2018.
- Deng L, Fang H, Tso VK, et al. Clinical validation of a novel urine-based metabolomic test for the detection of colonic polyps on Chinese population. Int J Colorectal Dis. 2017;32(5):741-743.
- Flexible sigmoidoscopy. Rochester (MN): Mayo Clinic; 2018: https://www.mayoclinic.org/tests-procedures/flexible-sigmoidoscopy/about/pac-20394189. Accessed 2019 Jan 25.
- Wang H, Tso V, Wong C, Sadowski D, Fedorak RN. Development and validation of a highly sensitive urine-based test to identify patients with colonic adenomatous polyps. Clin Transl Gastroenterol. 2014;5:e54.
- Riemann JF, Schroder C, Kallenbach M, Giersiepen K, Schmoll HJ. Benefits and risks of colorectal cancer screening. Oncol Res Treat. 2014;37 Suppl 3:11-20.
- Gudivada V, Apon A, Ding J. Data quality considerations for big data and machine learning: going beyond data cleaning and transformations. Int J Adv Software. 2017;10.
- Point of care, real-time urine metabolomics test to diagnose colorectal cancers and polyps in low- and middle-income countries. In: NIH Research Portfolio Online Reporting Tools (RePORT). Washington (DC): U.S. Department of Health & Human Services: https://projectreporter.nih.gov/project_info_description.cfm?aid=9221852&icde=0. Accessed 2019 Jan 25.
- C-Scan®: a new vision for colorectal cancer prevention. Isfiya (IL): Check-Cap; 2018: https://www.check-cap.com/. Accessed 2019 Jan 25.
- Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 2017;37(2):505-515.
- Alberta to begin assessing a new colorectal cancer screening strategy using Metabolomic Technologies’ PolypDx™ [news release]. Los Angeles (CA): Globe Newswire; 2018: https://globenewswire.com/news-release/2018/10/22/1624637/0/en/Alberta-to-begin-assessing-a-new-colorectal-cancer-screening-strategy-using-Metabolomic-Technologies-PolypDx.html. Accessed 2019 Jan 25.
A Robotic Exoskeleton for Gait Rehabilitation After Stroke
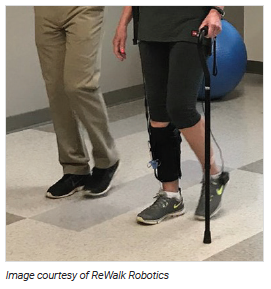 Stroke rehabilitation aims to reduce disability and help patients regain their ability to carry out the activities of daily living — one of the foundations of which is walking.1-3 Combining manually guided therapies with robot-assisted rehabilitation may reduce the fatigue of the therapist and ensure exercises are provided in a controlled manner and at an optimal level.4
Stroke rehabilitation aims to reduce disability and help patients regain their ability to carry out the activities of daily living — one of the foundations of which is walking.1-3 Combining manually guided therapies with robot-assisted rehabilitation may reduce the fatigue of the therapist and ensure exercises are provided in a controlled manner and at an optimal level.4
How It Works
There are two main types of robotic, or electromechanical-assisted, gait therapy systems:
- end-effector systems, such as the G-EO System (REHA Technology), and the Gait Trainer GT1 (Reha-Stim Medtec AG), which move the patient’s feet using foot plates2,5,6
- exoskeleton systems, such as the Lokomat (Hocoma), which move the hips, knees, and ankles.6-8
Established robotic exoskeleton gait systems are based on workstations that include treadmills with overhead body weight support and bilateral exoskeletons.6,9 Newer, mobile exoskeletons can be used with or without body weight support or a treadmill.10 The new generation exoskeletons, or “exosuits,” are intended to provide assistance for specific areas of weakness.11
The ReStore soft exosuit is a lightweight garment worn unilaterally on the affected leg, with a fabric sleeve that wraps around the calf and an insole under the foot. The leg and foot components are attached by cables to an activation unit and battery pack that can be worn around the waist for overground walking or placed adjacent to a treadmill. The ReStore can be used for both tethered (where the patient is connected to a system that supports their body weight during treadmill training) and untethered rehabilitation exercises.12,13 The device has built-in sensors to detect load and force of delivery, and the activation unit motor provides power to the weakened leg to assist in bending the ankle, raising the foot up and down for ground clearance (dorsiflexion and plantar flexion), and propelling the leg forward for walking.12
Who Might Benefit?
Stroke is a leading cause of death and long-term disability in Canada.14 Each year, more than 62,000 Canadians experience a stroke, and about 405,000 Canadians are living with the after-effects of stroke.15 Following a stroke, most patients will have some functional disabilities, many of which will improve within six months; however, in many patients, functional impairment, including difficulty walking, will persist beyond this period.2,16 As Canada’s population ages and new treatments improve survival rates, it is estimated that the number of people living with disability due to stroke will increase to 726,000 throughout the next 20 years.10,14,15
Availability in Canada
The ReStore exoskeleton (ReWalk Robotics, Inc.) is not currently commercially available.17 ReWalk Robotics first plans to apply for regulatory clearance in Europe and the US, where it anticipates ReStore may be commercially available in the first-half of 2019.17
What Does It Cost?
The different capabilities of current gait rehabilitation systems compared with the ReStore exoskeleton are not yet clear. A 2018 news item on the ReStore exoskeleton noted the company plans to price the system at under US$20,000.18 Based on the very limited cost information available, this may be less than the cost of other exoskeletons used in rehabilitation.19-21
Current Practice
Lower limb rehabilitation involves repetitive, task-oriented movements to improve strength and coordination.4 The focus is on relearning to walk using a combination of treadmill walking (with or without body weight support), community-based (overground) walking, and intensive repetition of functional mobility exercises, such as getting up from a chair.3 Rehabilitation is supervised by trained therapists, but the combination and application of exercises and the duration of therapy varies.3,4
Rehabilitation may differ in the subacute (within the first three to six months following a stroke) versus chronic (beyond six months) periods of rehabilitation.2 In the sub-acute phase, rehabilitation may focus on restoring the ability to walk independently, whereas the chronic phase may focus on improving walking ability.10 Neuroplasticity enabling recovery is believed to be greater during the sub-acute period.8,22
Canadian stroke rehabilitation guidelines recommend that robotic-assisted gait training may be used to complement, but not replace, conventional gait therapy, and should be considered for patients who might otherwise not practice walking.1
Published Studies
Results of three small, US, pilot, uncontrolled before-and-after studies of the ReStore exosuit were published in 2017 to 2018.12,13,23 These studies likely all used the same group of patients (a total of nine patients). The studies assessed the impact of the ReStore exosuit on walking speed, energy cost, muscle activity, forward propulsion, and ankle flexion for ground clearance.
Several recent systematic reviews of robotic-assisted gait training in stroke rehabilitation have been published but studies of the ReStore exoskeleton were not included.6,8,22,24,25
Safety
No studies on the safety of ReStore were identified. A single-arm, open-label safety study of ReStore involving 40 participants at five US rehabilitation hospitals was expected to be completed in November 2018.17,26 Each participant used the ReStore exoskeleton during a period of four weeks as part of their rehabilitation program.26
The primary outcome was the incidence of device-related adverse events, and secondary outcomes were the number of device malfunctions, injury to the therapist caused by the device, gait-related measures, and patient and therapist satisfaction.26 Study results are not yet available.
Issues to Consider
Robot-assisted gait training provides quantitative data on patient performance, such as walking speed and muscle strength, which may not otherwise be available.8,9
Older treadmill-based robotic systems may not be as beneficial as exoskeletons that allow overground walking — which is more challenging and demands more balance and control on the part of the user.9,10,22 Whether there are advantages to any particular exoskeleton system is not yet known and comparative studies are needed. There is also a lack of evidence on the optimal duration of training; whether benefits are maintained in the long term; and the impact on quality of life, activities of daily living, and falls.6,25
Other considerations include:
- the features of the different exoskeletons
- the type, level, and variability of assistance they provide
- whether the assistance is provided at the hip, knee, or ankle (or a combination of all)
- how the step is triggered
- the level of strength required by the patient
- whether the gait assistance is motor-driven or provided through functional electrical stimulation (Vickie Buttar, Glenrose Rehabilitation Hospital, Edmonton, AB: personal communication, 2019 Jan 28)
More evidence is needed on the impact of robotic exoskeletons on therapist fatigue and injury, and whether they can provide more intensive rehabilitation therapy (for example, a higher number of repetitions in a more accurate gait pattern). It has yet to be determined whether robotic systems can reduce the number of therapists needed during a rehabilitation session or increase the number of patients who can be treated during a session.
Related Developments
Examples of other mobile exoskeletons in use or under investigation for lowerbody stroke rehabilitation include EksoGT (Ekso Bionics, US), Indego Therapy (Parker Hannifin, US), and HAL (Cyberdyne, Japan).2,4,7,27
In January 2018, the US FDA expanded approved indications for the Indego exoskeleton to include the treatment of lower-limb paralysis due to stroke.28,29 The Exoskeleton for Post-Stroke Recovery of Ambulation (ExStRA) study, underway at three Canadian centres, is assessing gait rehabilitation outcomes in 40 patients using the EksoGT system.30 Other exoskeletons are being studied as a way to reduce musculoskeletal workplace injuries.31
Looking Ahead
A systematic review of the economic costs of robotic rehabilitation in adult stroke patients is underway at the Joanna Briggs Institute in Australia.32
The ReStore developers are investigating further uses of the exoskeleton for other conditions that affect mobility, such as multiple sclerosis and Parkinson disease.33 A multi-centre clinical study of ReStore in stroke rehabilitation is underway at five US centres.34
Author: Leigh-Ann Topfer
References
- Hebert D, Lindsay MP, McIntyre A, Kirton A, Rumney PG, Bagg S, et al. Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11(4):459-484.
- Bruni MF, Melegari C, De Cola MC, Bramanti A, Bramanti P, Calabro RS. What does best evidence tell us about robotic gait rehabilitation in stroke patients: A systematic review and meta-analysis. J Clin Neurosci. 2018;48:11-17.
- Eng JJ, Tang PF. Gait training strategies to optimize walking ability in people with stroke: A synthesis of the evidence. Expert Rev Neurother. 2007;7(10):1417-1436.
- Bortole M, Venkatakrishnan A, Zhu F, Moreno JC, Francisco GE, Pons JL, et al. The H2 robotic exoskeleton for gait rehabilitation after stroke: Early findings from a clinical study. J Neuroeng Rehabil. 2015;12:54.
- Wiener J, Foley N, Peireira S, Cotoi A, Chow J, Janssen S, et al. Lower extremity interventions. Evidence-based review of stroke rehabilitation. London (ON): Heart & Stroke Foundation, Canadian Partnership for Stroke Recovery; 2018: http://www.ebrsr.com/sites/default/files/v18-SREBR-CH9-NET-1.pdf. Accessed 2019 Jan 22.
- Mehrholz J, Pohl M, Kugler J, Elsner B. The improvement of walking ability following stroke: A systematic review and network meta-analysis of randomized controlled trials. Deutsches Arzteblatt international. 2018;115(39):639-645.
- Dierick F, Dehas M, Isambert JL, Injeyan S, Bouche AF, Bleyenheuft Y, et al. Hemorrhagic versus ischemic stroke: Who can best benefit from blended conventional physiotherapy with robotic-assisted gait therapy? PLoS One. 2017;12(6):e0178636.
- Cho JE, Yoo JS, Kim KE, Cho ST, Jang WS, Cho KH, et al. Systematic review of appropriate robotic intervention for gait function in subacute stroke patients. BioMed research international. 2018;2018:4085298.
- Weber LM, Stein J. The use of robots in stroke rehabilitation: A narrative review. NeuroRehabilitation. 2018;43(1):99-110.
- Louie DR, Eng JJ. Powered robotic exoskeletons in post-stroke rehabilitation of gait: A scoping review. J Neuroeng Rehabil. 2016;13(1):53.
- Weintraub K. Next-generation exoskeletons help patients move. The Scientist: Exploring Life, Inspiring Innovation 2018; https://www.the-scientist.com/features/next-generation-exoskeletons-help-patients-move-30126. Accessed 2019 Jan 28.
- Awad LN, Bae J, O’Donnell K, et al. A soft robotic exosuit improves walking in patients after stroke. Sci Transl Med. 2017;9(400).
- Sloot L, Bae J, Baker L, O'Donnell K, Menard N, Porciuncula F, et al. O 089 - A soft robotic exosuit assisting the paretic ankle in patients post-stroke: Effect on muscle activation during overground walking. Gait Posture. 2018;26:26.
- Stroke in Canada: Highlights from the Canadian Chronic Disease Surveillance System. Ottawa (ON): Public Health Agency of Canada; 2017: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/diseases-conditions/stroke-vasculaires/stroke-vasculaires-canada-eng.pdf. Accessed 2018 Dec 17.
- Lives disrupted: The impact of stroke on women. Toronto (ON): Heart and Stroke Foundation of Canada; 2018: https://www.heartandstroke.ca/-/media/pdf-files/canada/stroke-report/strokereport2018.ashx. Accessed 2018 Dec 13.
- Krueger H, Koot J, Hall RE, O'Callaghan C, Bayley M, Corbett D. Prevalence of individuals experiencing the effects of stroke in Canada: Trends and projections. Stroke. 2015;46(8):2226-2231.
- ReWalk launches clinical study for its ReStore soft exo-suit system [press release]. Marlborough, MA: ReWalk Robotics2018.
- Faulkner S. ReWalk Robotics ReStore soft exoskeleton expands clinical trials. The Robot Report 2018; https://www.therobotreport.com/rewalk-robotics-restore-exoskeleton-trials/. Accessed 2018 Dec 22.
- Ekso exoskeleton for rehabilitation in people with neurological weakness or paralysis. (Medtech innovation briefing MIB93). London (GB): National Institute for Health and Care Excellence; 2017: https://www.nice.org.uk/advice/mib93. Accessed 2018 Dec 22.
- Robotic treatment for stroke. Vancouver (BC): Vancouver Coastal Health; 2018: http://www.vch.ca/about-us/news/robotic-treatment-for-stroke. Accessed 2018 Dec 12.
- Marinov B. Reducing the cost of exoskeleton devices. Exoskeleton Report 2015; https://exoskeletonreport.com/2015/09/reducing-the-cost-of-exoskeleton-devices/. Accessed 2018 Dec 22.
- Schroder J, Truijen S, Van Criekinge T, Saeys W. Feasibility and effectiveness of repetitive gait training early after stroke: A systematic review and meta-analysis. J Rehabil Med. 2018.
- Bae J, Awad LN, Long A, O'Donnell K, Hendron K, Holt KG, et al. Biomechanical mechanisms underlying exosuit-induced improvements in walking economy after stroke. J Exp Biol. 2018;221(Pt 5).
- Lo K, Stephenson M, Lockwood C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients: A systematic review. JBI Database System Rev Implement Rep. 2017;15(12):3049-3091.
- Mehrholz J, Thomas S, Werner C, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2017;5:Cd006185.
- ReWalk Robotics Inc. NCT03499210: Safety evaluation of the ReWalk ReStore device in subjects with mobility impairments due to stroke. Clinicaltrials.gov. Bethesda (MD): US National Library of Medicine; 2018: https://clinicaltrials.gov/ct2/show/NCT03499210. Accessed 2018 Dec 22.
- Mizukami M, Yoshikawa K, Kawamoto H, Sano A, Koseki K, Asakwa Y, et al. Gait training of subacute stroke patients using a hybrid assistive limb: A pilot study. Disabil. 2017;12(2):197-204.
- Re: K173530. Trade/Device Name: Indego. Rockville (MD): U.S. Food & Drug Administration; 2018: https://www.accessdata.fda.gov/cdrh_docs/pdf17/K173530.pdf. Accessed 2018 Dec 11.
- FDA approves Indego Exoskeleton for stroke rehabilitation and treatment. Shepherd Center. 2018; https://news.shepherd.org/fda-approves-indego-exoskeleton-for-stroke-rehabilitation-and-treatment/. Accessed 2018 Dec 12.
- University of British Columbia. NCT02995265: Exoskeleton for post-stroke recovery of ambulation (ExStRA). ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2017: https://clinicaltrials.gov/ct2/show/NCT02995265. Accessed 2018 Dec 14.
- Goode L. Are exoskeletons the future of physical labor? The Verge. 2017; https://www.theverge.com/2017/12/5/16726004/verge-next-level-season-two-industrial-exoskeletons-ford-ekso-suitx. Accessed 2018 Dec 5.
- Lo K, Stephenson M, Lockwood C. The economic cost of robotic rehabilitation for adult stroke patients: A systematic review protocol. JBI Database System Rev Implement Rep. 2018;16(8):1593-1598.
- Melao A. ReStore exosuit could help MS patients improve their walking ability, study reports. Multiple Sclerosis News Today. 2017; https://multiplesclerosisnewstoday.com/2017/08/07/study-suggests-that-the-restore-exosuit-can-improve-ms-patients-ability-to-walk/. Accessed 2018 Dec 20.
- ReWalk Robotics Ltd. ReWalk announces progress in clinical study of ReStore powered exo-suit. 2018; https://www.prnewswire.com/news-releases/rewalk-announces-progress-in-clinical-study-of-restore-powered-exo-suit-300673647.html. Accessed 2019 Jan 22.
Mini-Roundup: Recent Reports From CADTH and Other Agencies
CADTH
Health Technology Update newsletter
Issues in Emerging Health Technologies bulletins
Recent Reports From Other Agencies
Agencies Included in the Mini-Roundup below:
- Cleveland Clinic Innovations, US
- Healthcare Improvement Scotland, Scottish Health Technologies Group (SHTG) National Health Service, National Services Scotland, UK
- The King’s Fund, UK
- National Institute for Health and Care Excellence (NICE), UK
- National Institute for Public Health and the Environment, the Netherlands
- Medgadget, US
Cardiovascular
Cancer, Imaging, and Radiology
- Colon Capsule Endoscopy (CCE-2) for the Detection of Colorectal Polyps and Cancer in Adults With Signs or Symptoms of Colorectal Cancer or at Increased Risk of Colorectal Cancer (SHTG)
- Outpatient Biopsy for Diagnosis of Suspicious Lesions of the Larynx, Pharynx, and Tongue Base (SHTG)
- Robot-Assisted Surgery Compared With Laparoscopic Resection for the Treatment of Rectal Cancer (SHTG)
Gastroenterology and Liver
Infectious Disease and Infection Control
Kidney and Urology
Nervous System and Neurology
Respiratory
- myAIRVO2 for the Treatment of Chronic Obstructive Pulmonary Disease (NICE)
- OxyMask for Delivering Oxygen Therapy (NICE)
- Servo-n With Neurally Adjusted Ventilatory Assist (NAVA) for Babies and Children (NICE)
- The Vest for Delivering High-Frequency Chest Wall Oscillation in People With Complex Neurological Needs (NICE)
- Video Laryngoscopes to Help Intubation in People With Difficult Airways (NICE)
Trends, Forecasts, and Strategic Initiatives
- Horizon Scan of Medical Technologies: Technologies with an Expected Impact on the Organisation and Expenditure of Healthcare (National Institute for Public Health and the Environment)
- Medgadget’s Best Medical Technologies of 2018 (Medgadget)
- Top 10 Medical Innovations for 2019(Cleveland Clinic)
- New Models of Home Care (The King’s Fund)
About This Document
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policymakers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein do not necessarily reflect the views of Health Canada, Canada’s provincial or territorial governments, other CADTH funders, or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Contact [email protected] with inquiries about this notice or legal matters relating to CADTH services.
ISSN: 1715-555X
